Solution: Draw the Lewis structure of xenon difluoride and give the number of lone pairs of electrons around the central atom 1.
following questions about atomic fluorine, oxygen, and xenon, as well as some of their provided, draw the complete Lewis electron-dot diagram for each of the. Lewis dot symbols provide a simple rationalization of why elements form compounds When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in Exercise \(\PageIndex{3}\): Xenon Difluoride.
Xenon (Xe). Diagram of the nuclear composition and electron configuration of an atom of xenon (atomic number: 54), the most common isotope of this.
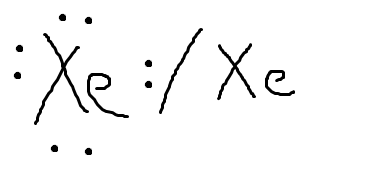
Xenon has 8 dots (4 pairs of dots) around the letters Xe.Comprehensive information for the element Xenon – Xe is provided by this page including scores of properties, element names in many languages, most known nuclides and . The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence electrons. Refer to the related link for an illu.
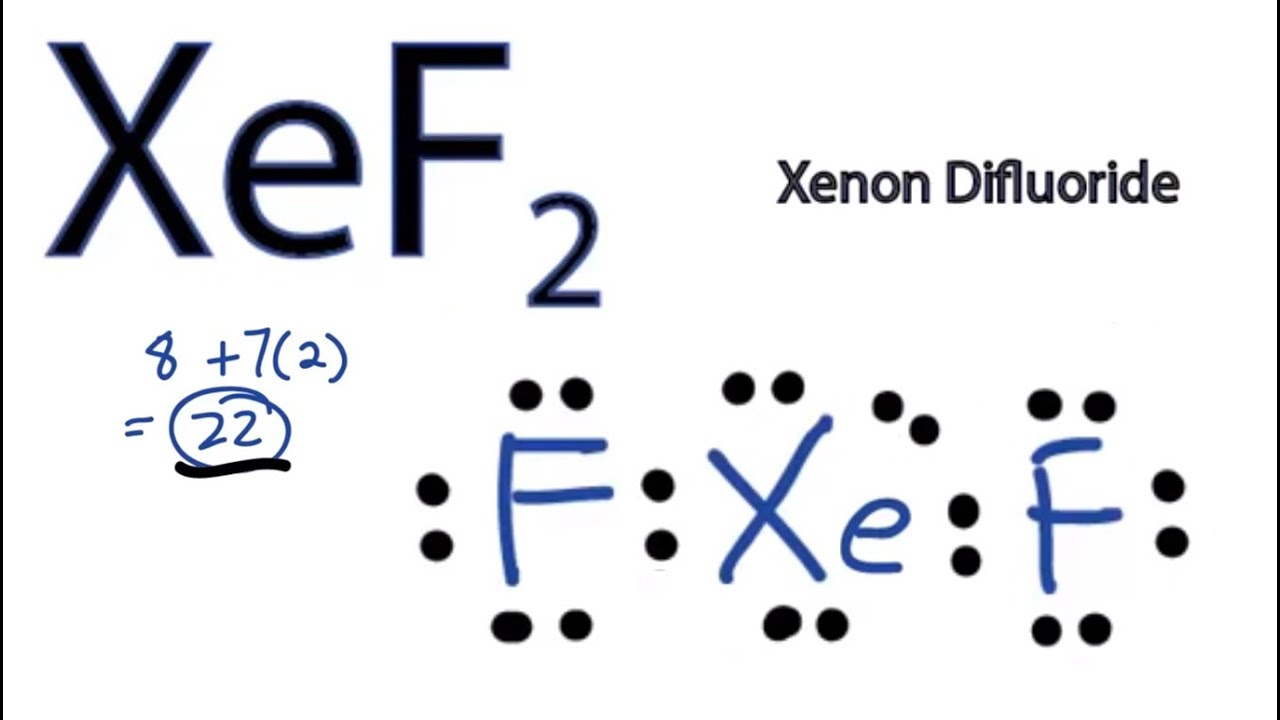
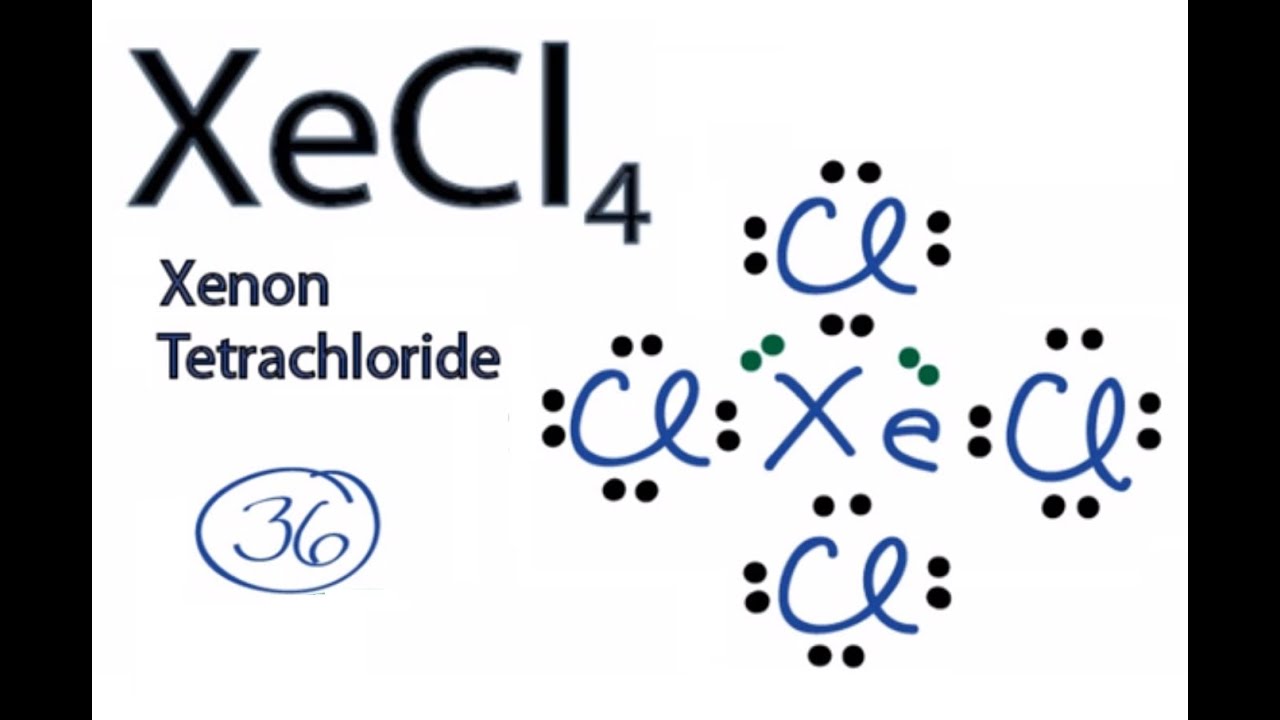
The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can .

Aug 17, · How do you draw an XeF2O Lewis Structure? hybridization of xenon. To create a double bond to oxygen we need a p orbital with just one electron in it and we need an sp [math]^2 [/math] How do you draw the Lewis dot structure of Y3B2?
How can I draw the Lewis dot structure of (NH4) 2Cr2O7 (Ammonium dichromate)?. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his article The Atom and the Molecule.
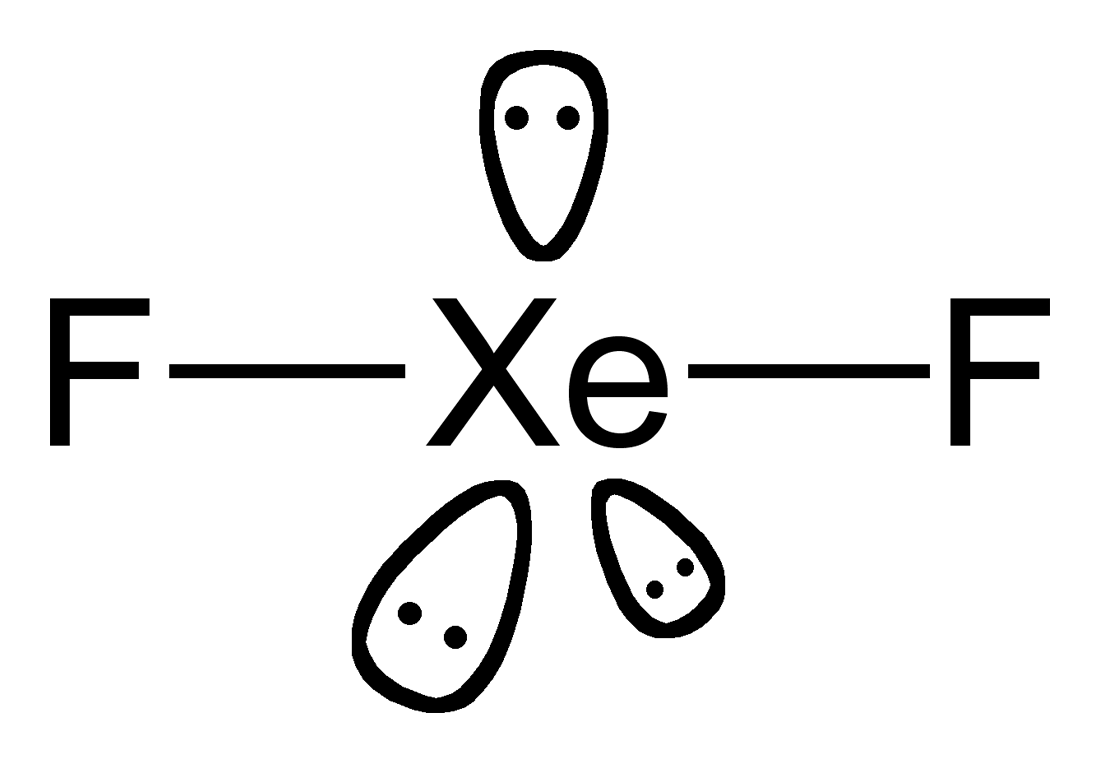
Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.Answer: Draw the Lewis structure of xenon | Clutch PrepLewis structure – Wikipedia