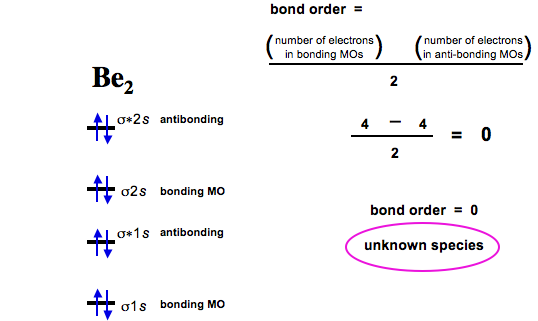
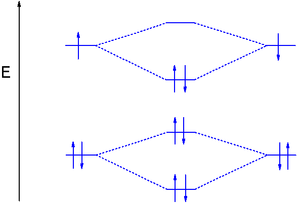
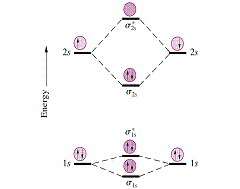
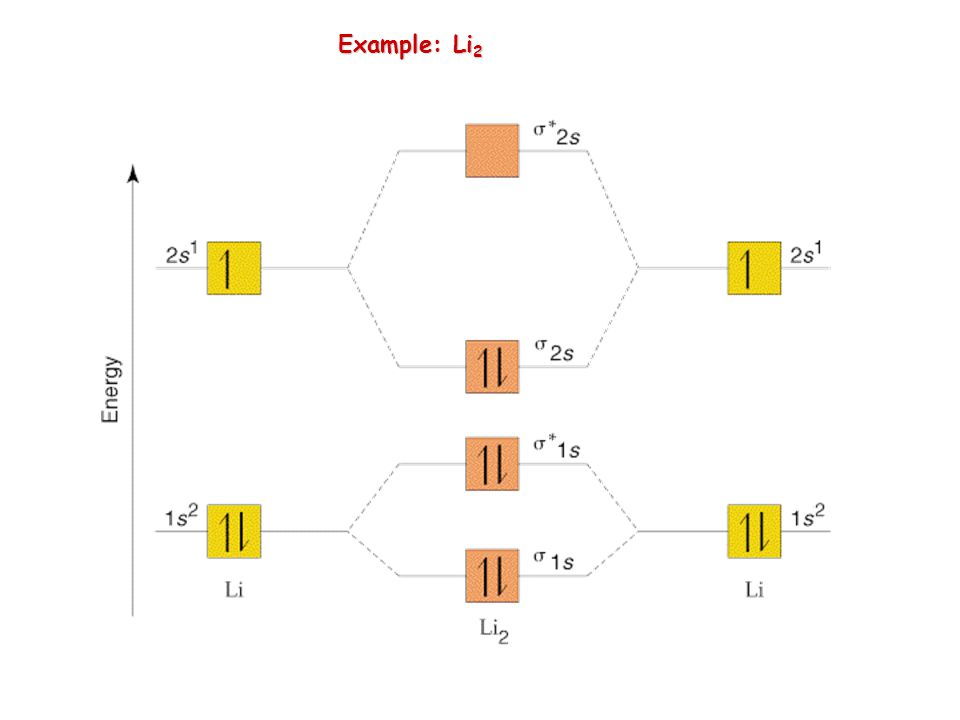
diagram; the MOs go between them. Consider the MO diagram for Li2.
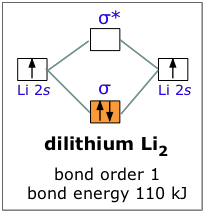
This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
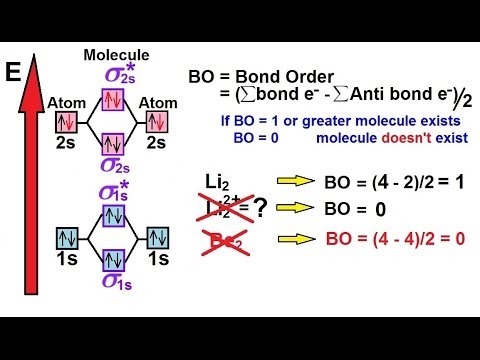
The molecule . Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6.
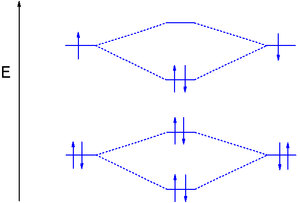
Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2.
B.O =(Nb- Na). B.O = (). B.O = 1.
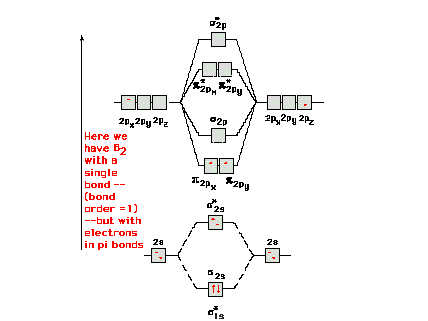
Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Answer to Draw a molecular orbital energy diagram for Li2. What is the bond order?
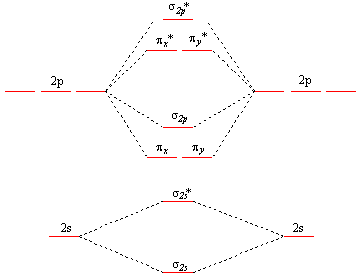
Is the molecule likely to be stable? Explain.Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2.
From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2?
Solution. We draw a molecular orbital energy diagram similar to that shown in Figure Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
The same method can be applied to for other diatomic molecules, but involving more than the 1 s atomic orbitals. Molecular orbitals: Molecular orbitals are formed by linear combination of atomic orbitals.
Atomic orbitals and molecular orbitals of a molecule can be shown in a diagram called as molecular orbital diagram. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +.
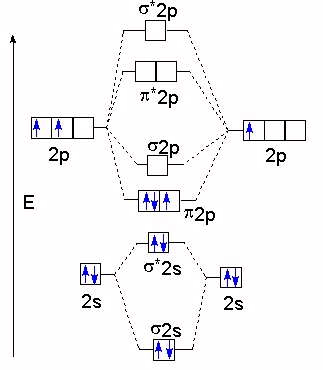
Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as . A molecule in which all the electrons are paired, is called diamagnetic while molecule which has one or more unpaired electron is called paramagnetic.
Molecular orbital diagram of H 2 (Hydrogen molecule).Molecular orbitals of diatomic molecules.Molecular Orbitals