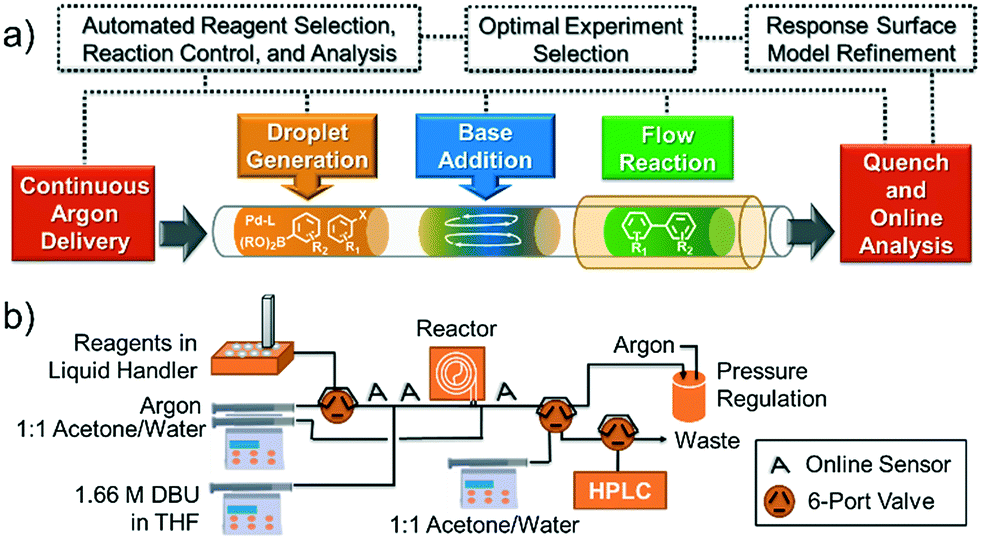
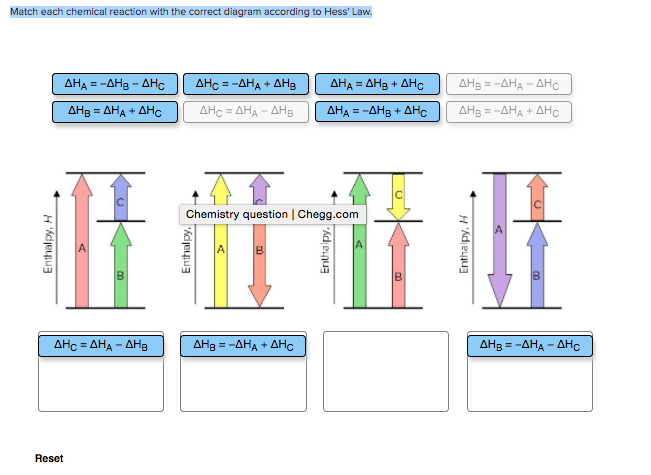
Answer to 13 Question 13 (of 15) points Match each chemical reaction with the correct diagram according to Hess Law. A Reset. Hess’ Law of Constant Heat Summation The enthalpy of a given chemical reaction is constant, regardless of the reaction happening in one step or many Another way to state Hess’ Law is: 1) Analyze what must happen to each equation.

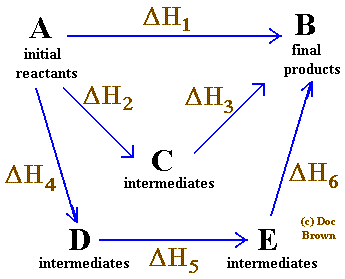
Hess’s Law is the most important law in this part of chemistry. If you look at the change on an enthalpy diagram, that is actually fairly obvious. This shows the enthalpy changes for an exothermic reaction using two different Law cycle, writing the known enthalpy changes over the arrows for each of the other changes.

A fire is started in a fireplace by striking a match and 8. exothermic?
Explain what Correct the incorrect statements and explain. 6. Hess’s law is really just another statement of the first law of thermodynamics.

Calculate DE, DH, q, and w for each case. the following potential energy diagrams for two different reactions. Based on the first law of thermodynamics, the energy gained by a system is ____ In the enthalpy diagram shown, the reactants are _____ in energy than the during a chemical reaction (discounting any small volume change caused by liquids or solids).
gas. Match each thermodynamic symbol with its correct definition.In a chemical reaction, the total mass of the products compared to the total mass of the reactants is: Moles. An excess of Al and mol of Br2 are reacted according to the equation 2Al + 3Br2 -> 2AlBr3 Hess’ Law.
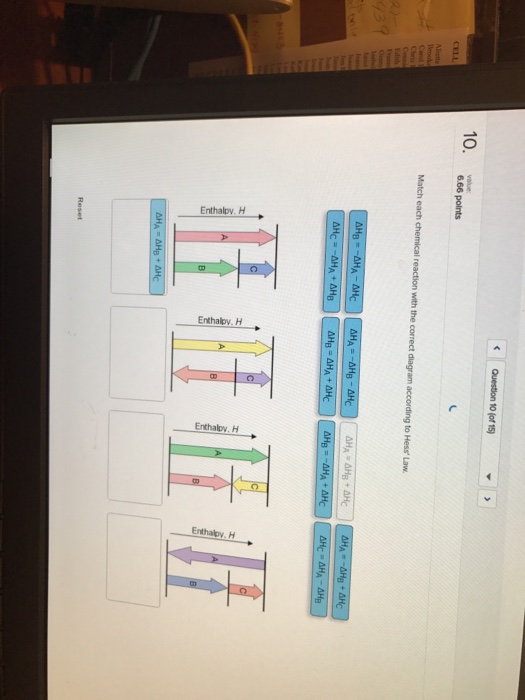
What principle relates the enthalpy change for a net reaction to the enthalpy changes of a series of summed reactions?. Question: Match each chemical reaction with the correct diagram according to Hess’ Law Match each chemical reaction with the correct diagram according to Hess’ Law Show transcribed image text Match each chemical reaction with the correct diagram according to Hess’ Law%(5).
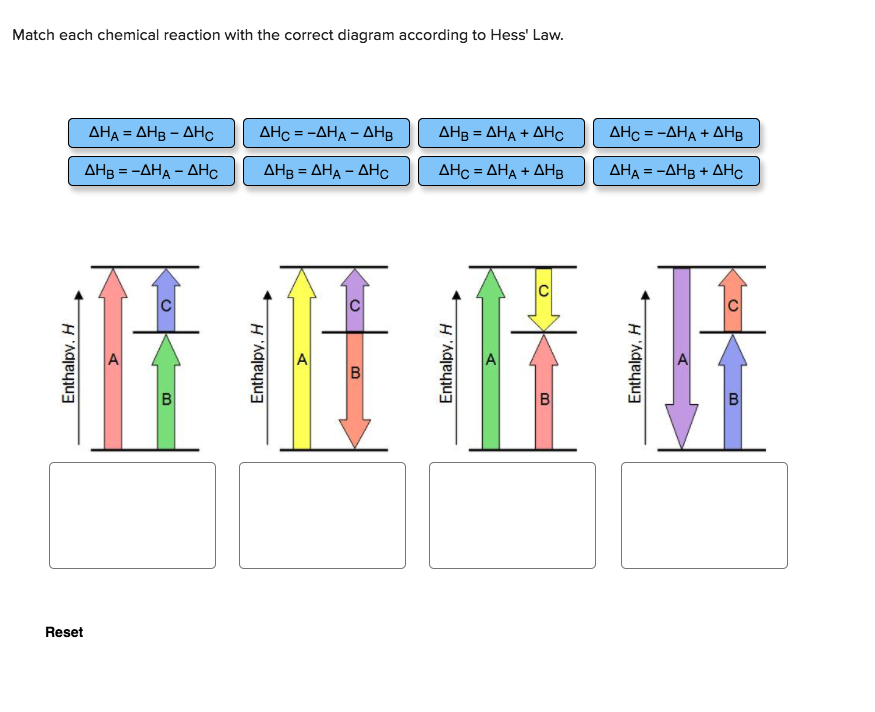
home / study / science / chemistry / chemistry questions and answers / Match Each Chemical Reaction With The Correct Diagram According To Hess Law. Delta H_A = -Delta Delta H_A = -Delta Question: Match each chemical reaction with the correct diagram according to Hess Law. Hess’s Law is the most important law in this part of chemistry. Most calculations follow from it. It says The enthalpy change accompanying a chemical change is independent of the route by which the chemical change occurs.
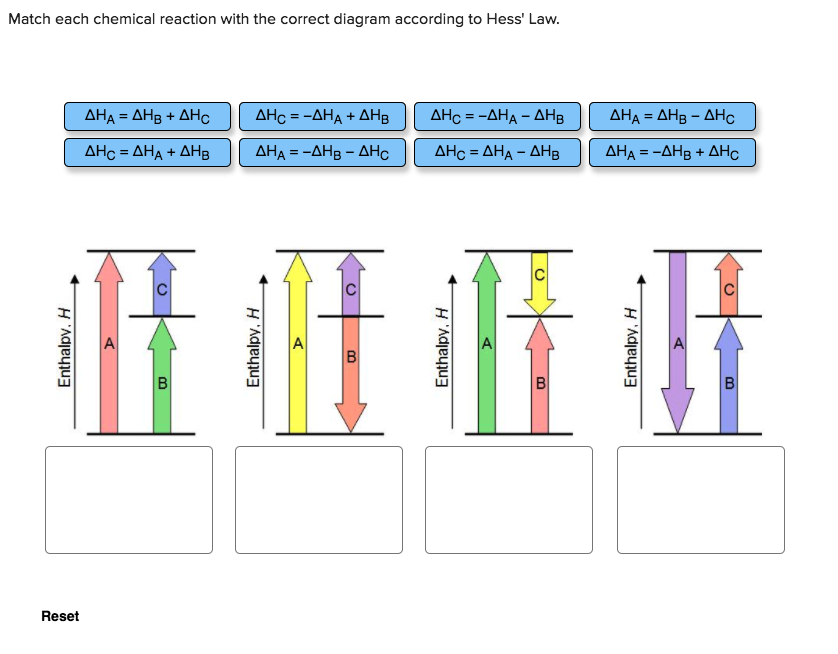
Hess’s Law is saying that if you convert reactants A into products B. Consider the following intermediate chemical equations. mcjpg In the final chemical equation, NaCl and Omcjpg are the products that are formed through the .ChemTeam: Hess’ Law – using three equations and their enthalpiesHess’s Law – Conservation of Energy
