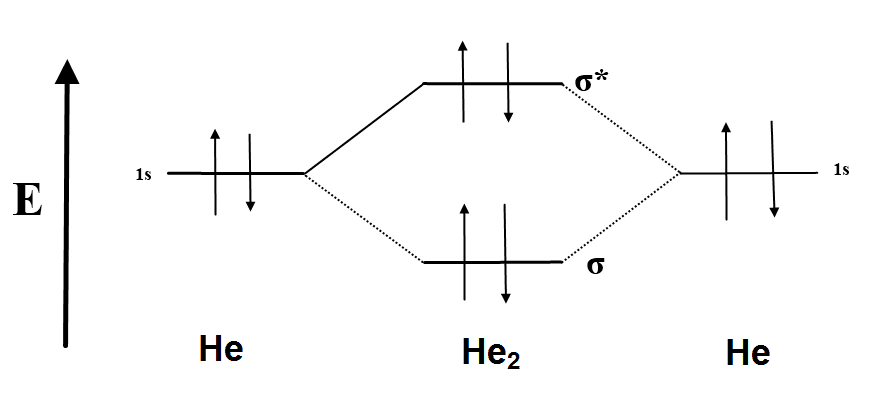
According to the molecular orbital theory, in a supposed He2 molecule, both the bonding and the antibonding orbitals will have 2 electrons each. And so, the. I have recently learned that according to molecular orbital theory (MOT), anti- bonding sigma* 1s shells exist (in terms of potential energy).
•Molecular orbital theory (MO) – a molecule is formed by the overlap of .. He2. + σ*1s σ1s.
E n e rg y. He2.
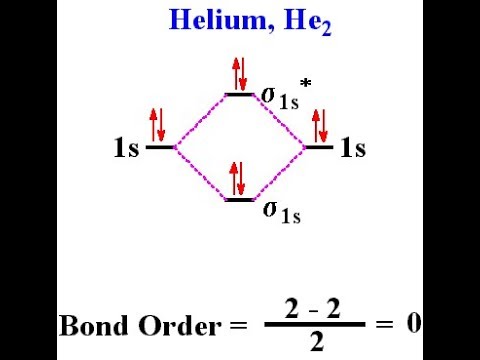
+ bond order =?? MO Diagram for He2.
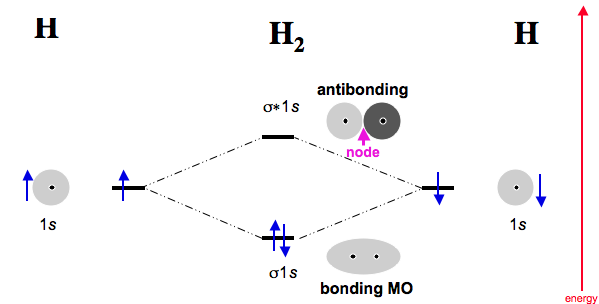
+ and H2. I have recently learned that according to molecular orbital theory (MOT), anti- bonding sigma* 1s shells exist (in terms of potential energy).
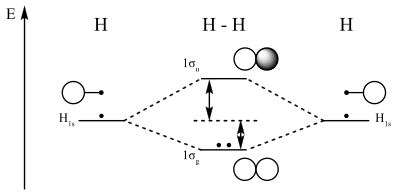
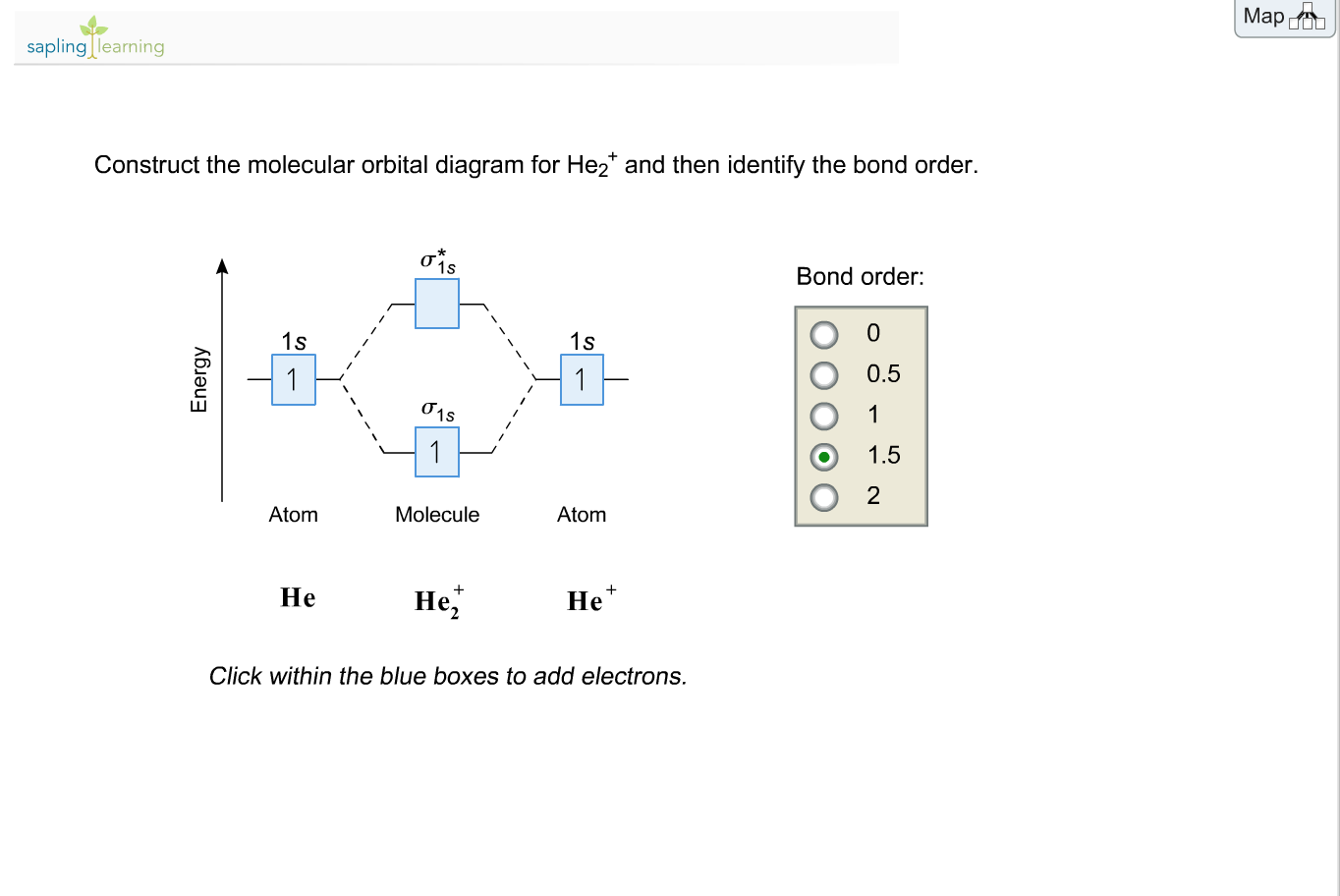
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe.The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in .
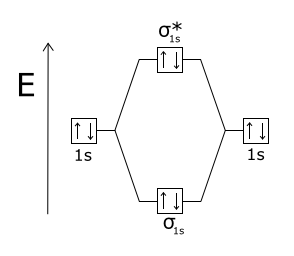
Jun 30, · It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4) Bond order 1. It is stable.
Chemical Forums
In fact, it’s the perioxide ion. Check me out: schematron.org The reason oh He2 Molecule to not exist can be explained on the basis of 1)MOLECULAR ORBITAL THEORY.
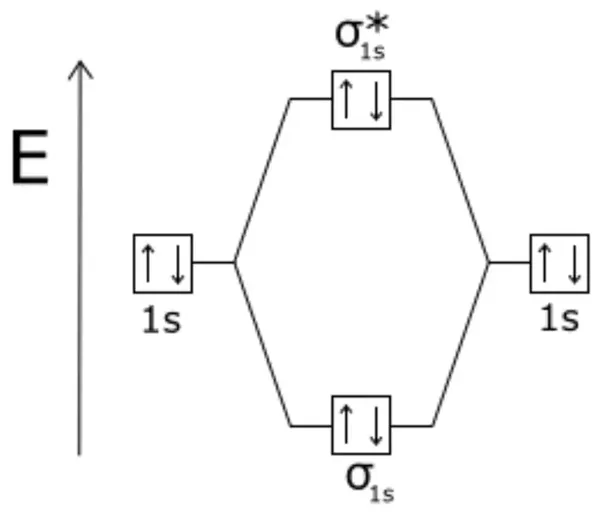
He has configuration of 1s2, if we draw its MOT DIAGRAM, 2 e’s enter the Bonding molecular Orbital and 2 e’s enter the AntiBonding molecular Orbital, thus net effect of the anti bonding and bonding is cancelled. A summary of Molecular Orbital Theory in ‘s Molecular Orbitals. Learn exactly what happened in this chapter, scene, or section of Molecular Orbitals and what it means.
Perfect for acing essays, tests, and quizzes, as well as for writing lesson plans. Please note the diagram is for He2+ but the He-H is very similar Eg: Li + H; Li has 1s + 2s, while H has 1s. This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s, and a non bonding orbital from Li1s (lower in energy than the sigma).Molecular orbital theory of He2SparkNotes: Molecular Orbitals: Molecular Orbital Theory