No+ is a cation that consists of one nitrogen atom (N) and one oxygen atom (O) with a positive charge. Understanding the molecular orbital (MO) diagram of No+ is crucial for understanding its electronic structure and bonding. MO theory is a powerful tool used to explain the behavior and properties of molecules based on the principles of quantum mechanics.
In the MO diagram of No+, the molecular orbitals are constructed by combining the atomic orbitals of the nitrogen and oxygen atoms. The nitrogen atom contributes three valence electrons, while the oxygen atom contributes four valence electrons. The total number of valence electrons in No+ is thus seven.
The MO diagram of No+ shows that the lowest energy level occupied by electrons is the sigma bonding molecular orbital (σ1s). Above this are two degenerate molecular orbitals: the sigma antibonding molecular orbital (σ*1s) and the pi bonding molecular orbital (π2p). The highest energy level is the pi antibonding molecular orbital(π*2p).
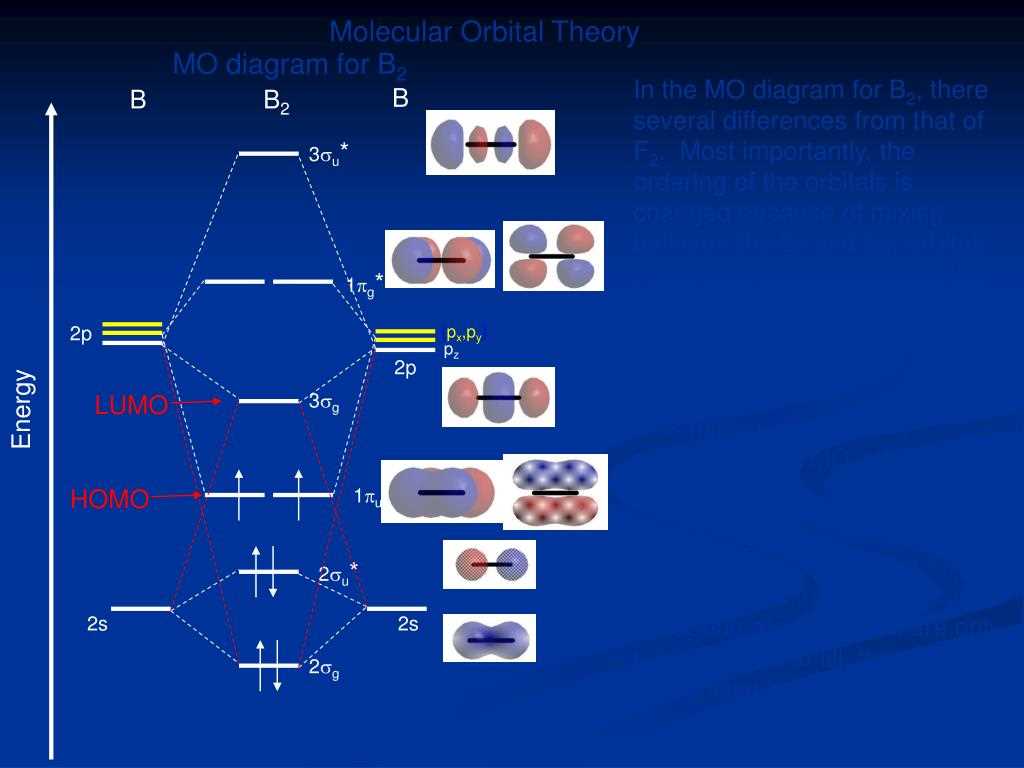
Mo diagram of NO+
The molecular orbital (MO) diagram of NO+ is used to illustrate the distribution and arrangement of electrons in the molecule. This diagram provides valuable information about the bonding and antibonding interactions between the atomic orbitals of nitrogen (N) and oxygen (O) atoms in the NO+ ion.
In the MO diagram of NO+, the atomic orbitals of nitrogen and oxygen are combined to form a set of molecular orbitals. The five atomic orbitals involved in the formation of these molecular orbitals are the 2s and 2p orbitals of nitrogen, and the 2s and 2p orbitals of oxygen. The diagrams highlight the energy levels and symmetries of these molecular orbitals.
The MO diagram of NO+ shows that there are a total of five molecular orbitals formed from the combination of atomic orbitals. These are labelled as σ2s, σ2s*, π2p and π2p*, and δ2p. The σ2s and π2p orbitals are bonding orbitals, while the σ2s* and π2p* orbitals are antibonding orbitals. The δ2p orbital is a nonbonding orbital.
The occupied orbitals are depicted by placing electrons in the molecular orbitals according to the Aufbau principle and the Pauli exclusion principle. The molecular orbitals are filled starting from the lowest energy level (σ2s) and progressing to the higher energy levels. In the case of NO+, the electrons occupy the σ2s, π2p, and δ2p orbitals, with a total of nine electrons in the system.
Overview
In the field of chemistry, molecular orbital (MO) theory is a widely-used approach to understand and describe the electronic structure of molecules. One specific application of MO theory is the construction of MO diagrams, which provide a visual representation of the energy levels and electron distribution in a molecule. In this context, the NO+ molecule, also known as the nitrosyl cation, is of particular interest.
The NO+ molecule consists of a nitrogen atom bonded to an oxygen atom, with a positive charge on the nitrogen atom. The MO diagram of NO+ is constructed by combining the atomic orbitals of the nitrogen and oxygen atoms, taking into account their relative energies and symmetries. The resulting MO diagram reveals the distribution of electrons in the molecule and helps to explain its stability and reactivity.
The MO diagram of NO+ shows a combination of sigma and pi bonding and antibonding orbitals. The nitrogen atom contributes its 2s and 2p orbitals, while the oxygen atom contributes its 2s and 2p orbitals as well. The sigma bonding orbital is formed by the overlap of the 2s orbitals of nitrogen and oxygen, while the pi bonding orbitals are formed by the overlap of the 2p orbitals. The antibonding orbitals are formed by the subtraction of the orbitals with opposite phases.
Overall, the MO diagram of NO+ provides insights into the electronic structure of the molecule and helps to explain its properties. By understanding the energy levels and electron distribution, scientists can make predictions about the behavior of NO+ in chemical reactions and its interaction with other molecules.
Electronic configuration of NO+
NO+ is the cationic form of the molecule nitric oxide. It consists of a nitrogen atom (N) and an oxygen atom (O) bonded together with a positive charge. To understand the electronic configuration of NO+, we need to analyze the valence electrons and molecular orbital diagram of the molecule.
The atomic number of nitrogen is 7, and oxygen is 8. Nitrogen contributes 5 valence electrons, while oxygen contributes 6 valence electrons. The total number of valence electrons in NO+ is therefore 11. In the neutral form of NO, there are 3 lone pairs of electrons on the nitrogen atom and 2 lone pairs on the oxygen atom. However, in NO+, one electron is removed, resulting in the loss of one lone pair from the nitrogen atom.
The molecular orbital diagram of NO+ shows that the nitrogen atom contributes 3 valence electrons to the bonding orbitals, while the oxygen atom contributes 2 valence electrons. These electrons are distributed into various molecular orbitals, including sigma (σ) and pi (π) orbitals. The σ bonding orbitals are formed by the overlap of atomic orbitals between nitrogen and oxygen, while the π bonding orbitals are formed by the side-to-side overlap of p orbitals.
The electronic configuration of NO+ can be represented as (σ2)(π4)(σ*2), where (σ2) represents the two electrons in the σ bonding orbitals, (π4) represents the four electrons in the π bonding orbitals, and (σ*2) represents the two electrons in the σ* antibonding orbitals. Overall, the electronic configuration of NO+ reflects the redistribution of electrons due to the removal of one electron, resulting in a positive charge on the molecule.
Symmetry Considerations
Symmetry considerations play a crucial role in understanding the electronic structure of molecules and determining their properties. In the case of the MO diagram of NO+, the use of symmetry allows us to simplify the calculation and get insights into the bonding and antibonding orbitals of the molecule.
The symmetry of a molecule is described by its point group, which is determined by its shape and arrangement of atoms. In the case of NO+, the molecule belongs to the Cs point group, which has a symmetry plane that bisects the molecule into two halves. This symmetry plane plays a key role in determining the symmetry of the molecular orbitals.
Using group theory, we can apply symmetry operations to the molecular orbitals to determine their symmetry properties. In the MO diagram of NO+, the sigma bonding orbital (σ) is formed by the overlap of the nitrogen-p atomic orbital and the oxygen-s atomic orbital. This bonding orbital has a symmetry that is consistent with the symmetry of the Cs point group.
In addition to the sigma bonding orbital, there are also other molecular orbitals in the MO diagram of NO+ that exhibit different symmetries. These include the sigma star antibonding orbital (σ*) and the pi bonding and antibonding orbitals (π and π*). By considering the symmetry properties of these orbitals, we can predict the stability and reactivity of the molecule.
In conclusion, symmetry considerations provide a powerful tool for understanding the electronic structure of molecules and determining their properties. In the case of NO+, the use of symmetry allows us to simplify the calculation of the MO diagram and gain insights into the bonding and antibonding orbitals. By considering the symmetry properties of these orbitals, we can better understand the stability and reactivity of the molecule.
Formation of Mo diagram
The molecular orbital (MO) diagram of NO+ is formed by combining the atomic orbitals of nitrogen and oxygen atoms. Nitrogen has three valence electrons, while oxygen has two, resulting in the formation of a cation with a positive charge (+1). The atomic orbitals involved in the formation of the MO diagram are the 2s and 2p orbitals of nitrogen, as well as the 2s orbital of oxygen.
The 2s orbital of nitrogen and the 2s orbital of oxygen overlap to form a sigma bonding MO. This bonding MO is lower in energy compared to the atomic orbitals, indicating a stabilizing interaction. The remaining two 2p orbitals of nitrogen and the 2p orbital of oxygen overlap to form two pi bonding MOs. These pi bonding MOs are also lower in energy compared to the atomic orbitals, further stabilizing the molecule.
Additionally, there are two antibonding MOs formed as a result of the overlap between the atomic orbitals. These antibonding MOs are higher in energy compared to the atomic orbitals and contribute to the destabilization of the molecule. The occupancy of the MOs follows the Aufbau principle, with the lower energy MOs filled first with electrons.
In summary, the formation of the MO diagram of NO+ involves the overlap of atomic orbitals from nitrogen and oxygen, resulting in the formation of bonding and antibonding MOs. The bonding MOs contribute to the stability of the molecule, while the antibonding MOs contribute to the destabilization. The occupancy of the MOs follows the Aufbau principle.
Interpretation of the Mo diagram
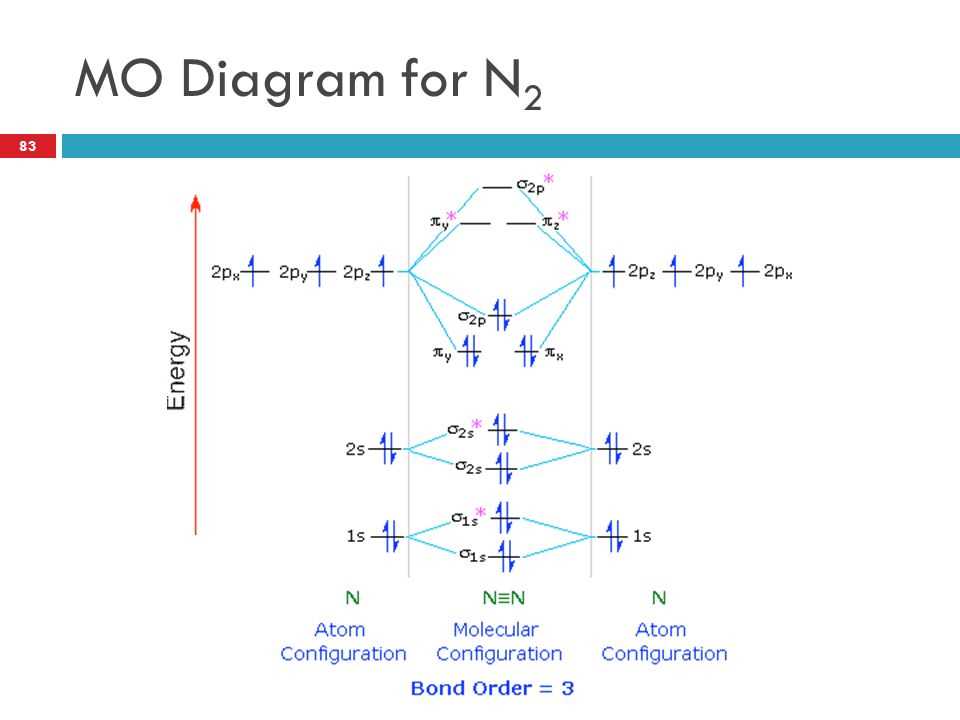
The molecular orbital (Mo) diagram provides a visual representation of the distribution of electrons in a molecule, specifically in the case of the NO+ ion. The Mo diagram for NO+ shows the energetic levels of the molecular orbitals and the occupancy of electrons in these orbitals.
The Mo diagram for NO+ consists of a total of 8 electrons, distributed among the various molecular orbitals. The diagram shows that the molecular orbitals are grouped into bonding and antibonding orbitals. The energy of the bonding orbitals is lower than that of the antibonding orbitals, which results in a stable molecule.
The highest occupied molecular orbital (HOMO) of NO+ is a pi orbital, which is important for the chemical reactivity of the molecule. The HOMO is responsible for the interaction with other molecules or ions during chemical reactions. In the case of NO+, the presence of a lone pair of electrons in the HOMO provides the molecule with its lewis basic character.
The Mo diagram also shows that there are unpaired electrons in the antibonding orbitals, which indicates that NO+ is a paramagnetic molecule. This means that it is attracted to a magnetic field due to the presence of unpaired electrons.
In summary, the Mo diagram for NO+ provides a comprehensive understanding of the electronic structure of the molecule. It reveals the bonding and antibonding orbitals, the occupation of electrons, and the presence of unpaired electrons. This information is crucial for understanding the chemical reactivity and properties of NO+.