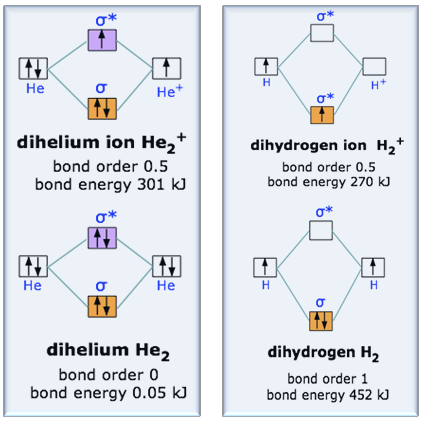
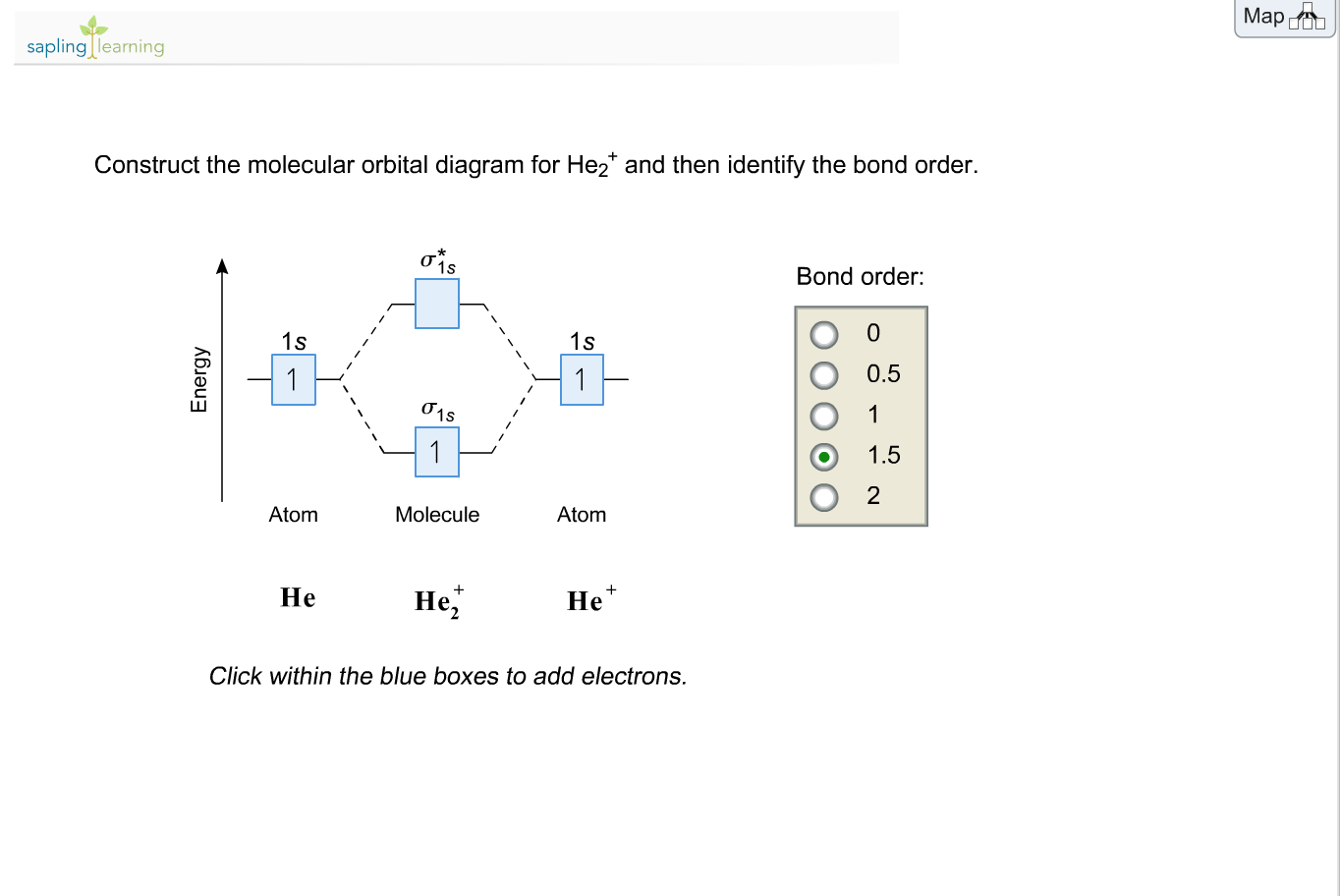
He2+ MO diagram. Eg: Li + H; Li has 1s + 2s, while H has 1s.
This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s. The bond order of a simple molecule can be determined by looking at the number of electrons in bonding and antibonding molecular orbitals. Like electrons in.
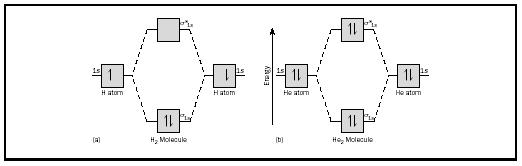
According to Molecular Orbital (MO) Theory, two atoms mix their orbitals to form one that is spread out over both Figure1: MO diagram for H2. After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide. Construct the molecular orbital diagram for He2+ and identify the bond order.
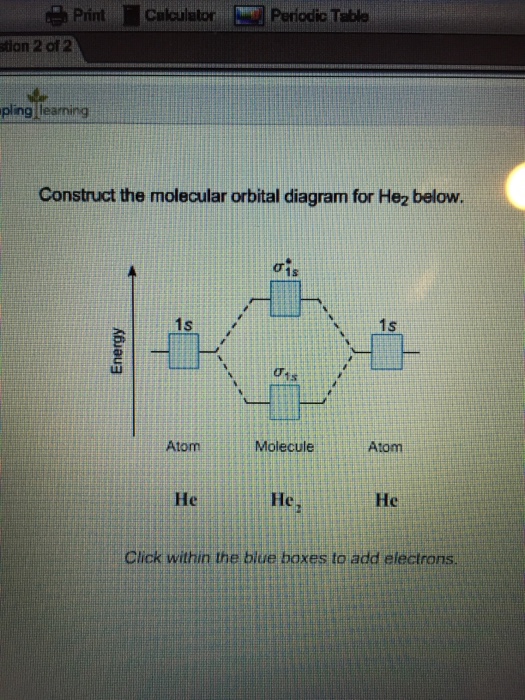
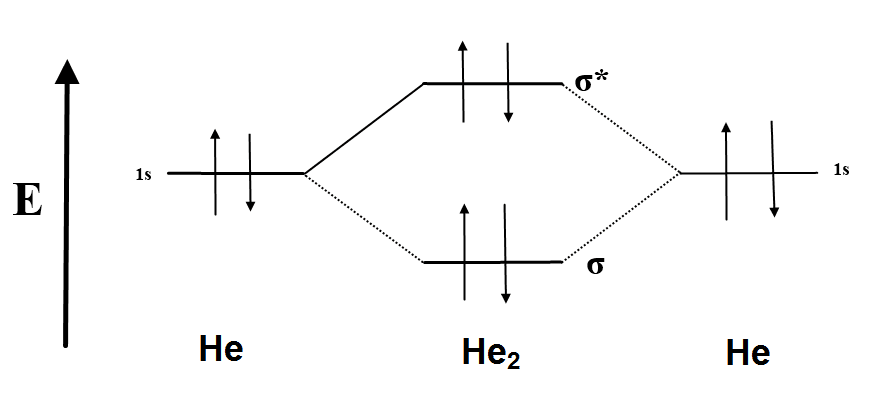
Question 7 of 16 Incorrect Map Sapling Learning macmillan learning Construct the.Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule. The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule.
Two are placed in the bonding orbital, the other two in antibonding orbital.
The bond order = 1/2 x (Number of Bonding Electrons – Number of Antibonding Electrons) = . The reason oh He2 Molecule to not exist can be explained on the basis of 1)MOLECULAR ORBITAL THEORY.

He has configuration of 1s2, if we draw its MOT DIAGRAM, 2 e’s enter the Bonding molecular Orbital and 2 e’s enter the AntiBonding molecular Orbital, thus net effect of the anti bonding and bonding is cancelled. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory.
The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below. The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding.Diatomic Species | MO theory | ChemogenesisMolecular Orbital Theory