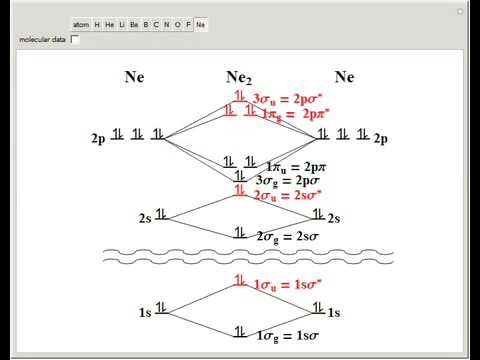
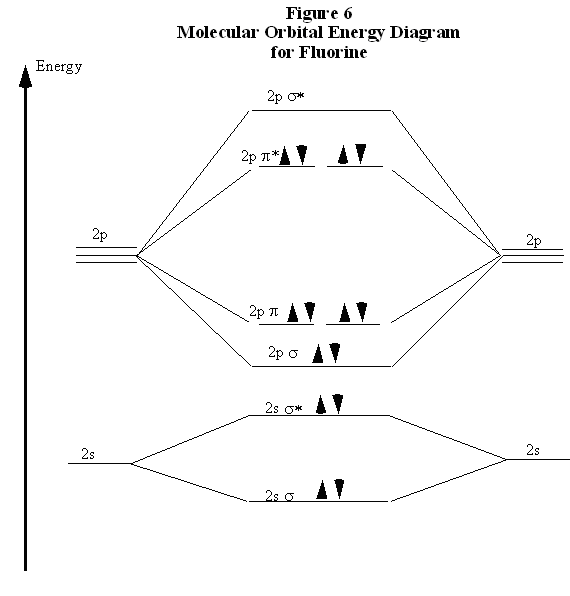
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.

Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2.
(Don’t memorize.) Li2 through N2. O2 through Ne2.
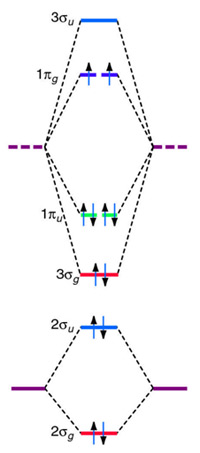
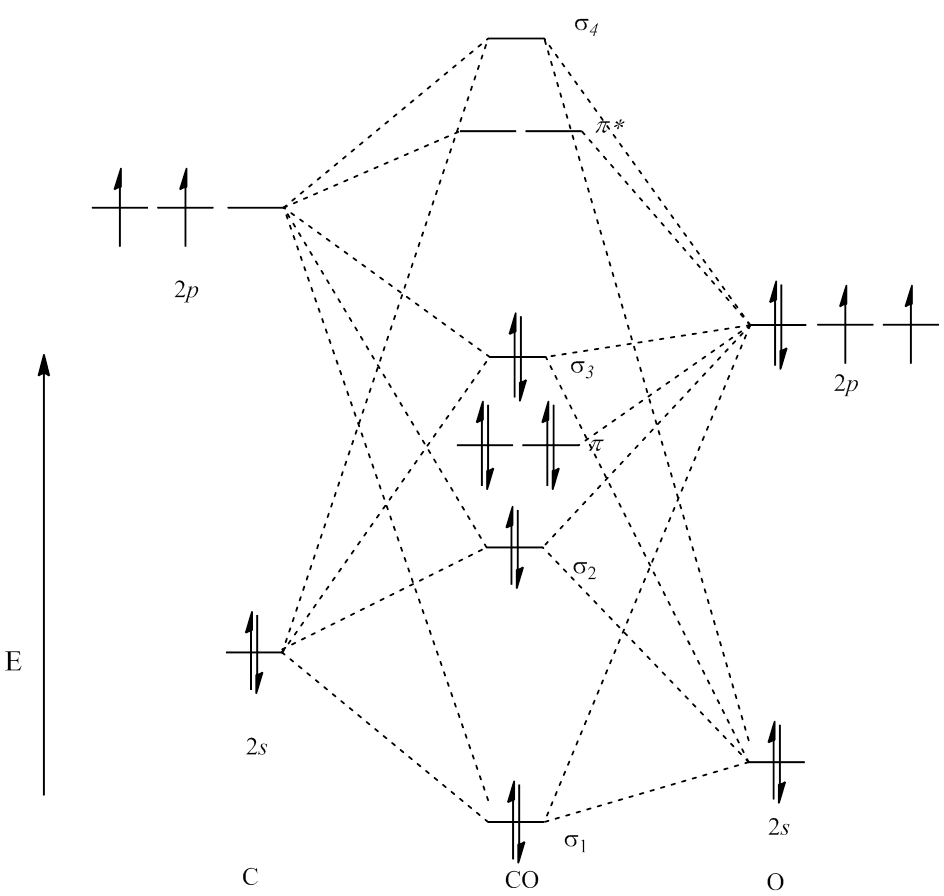
After reading the theory part draw the MO diagrams for the following diatomic omonuclear molecules: H2, B2, C2, N2, O2, Ne2, F2 choosing the correct. This Videos are recorded in live lectures by 20 years experienced Chemistry teacher, to help students to understand concepts in much better.Each valence shell has one 2s and three 2p orbitals, and so there are eight atomic orbitals in all and hence eight molecular orbitals that can be formed.
The energies of these atomic orbitals are shown on either side of the molecular orbital energy-level diagram in Figure The short answer is: we could not tell it using the primitive molecular orbital theory introduced in the general chemistry courses.
In exact same way we could not tell why $\mathrm{\sigma_{2p_{z}}}$ MO becomes lower in energy than $\mathrm{\sigma_{2p_{z}}}$ MO to the left of $\ce{N2}$ and not to the left of, say, $\ce{C2}$. Forming Molecular Orbitals. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule.
Consider the H 2 molecule, for example. One of the molecular orbitals in this molecule is constructed by adding the mathematical functions for the two 1s atomic orbitals that come together to form this molecule. Another orbital is formed by subtracting one of these functions from the .
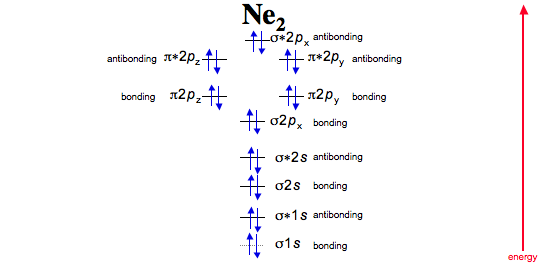
For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 schematron.org each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order%(1).
The bond order is the difference in the number of electron pairs occupying an antibonding and a bonding molecular orbital. Because hydrogen has one electron pair in its bonding orbital and none in its antibonding orbital, molecular orbital theory predicts that H 2 has a bond order of one–the same result that is derived from Lewis structures.Diatomic Species | MO theory | ChemogenesisMolecular Orbital Theory