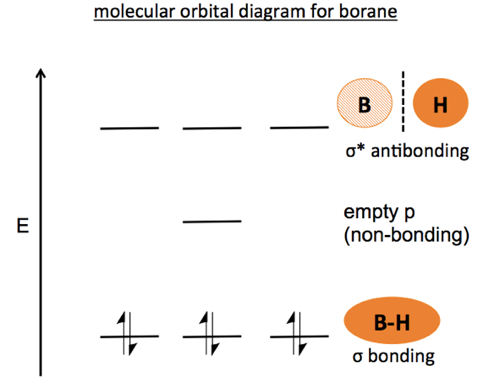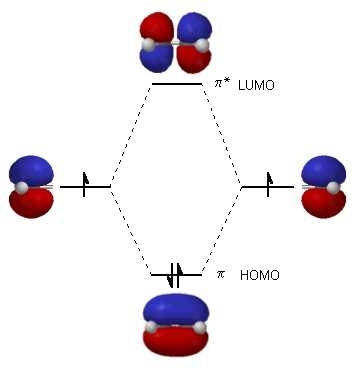
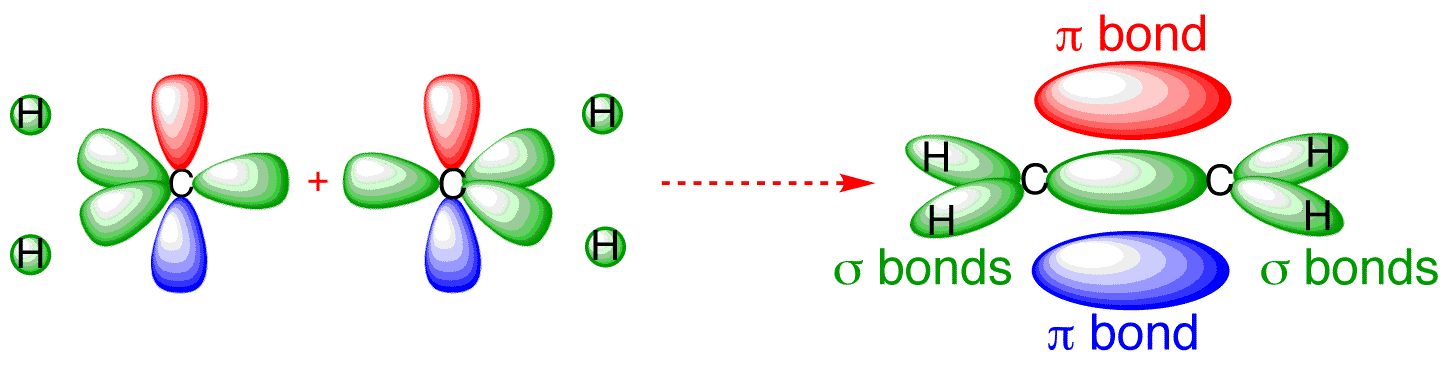
Each line in this diagram represents one pair of shared electrons. Ethene This sideways overlap also creates a molecular orbital, but of a different kind. In this.
Sigma (σ) bonding molecular orbital – Shared electron density is directly .. Problem 6 – Use ethene (H2C=CH2) as a model to draw an MO diagram for ethanal. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction .
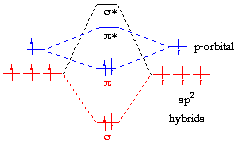
For the ethene orbital energy diagram these are shown as pCC for the. Molecular Orbital Analysis of Ethene Dimerisation π Molecular Orbitals of 1,3- Butadiene essentially the same theory about how acids and bases behave. π Molecular Orbitals of Ethene The diagram to the right shows the relative energies of the atomic p orbitals, the resulting π molecular orbitals and the electron.That photograph (Orbital Diagram Pretty Ethene Molecular orbitals Images) preceding will be classed with:put up through Diana Collins with To find out all pictures with New Photograph Of orbital Diagram photographs gallery make sure you follow this website link.
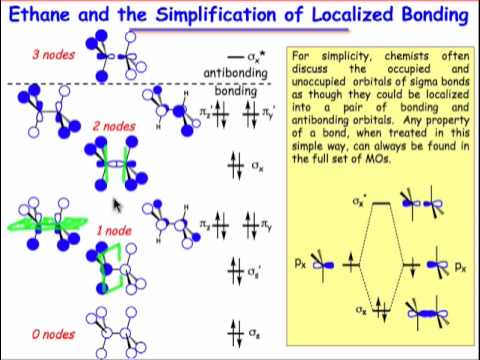
Sigma (σ) bonding molecular orbital – Shared electron density is directly between the bonding atoms, along the bonding axis. A sigma bonds is always the first bond formed between two atoms. The molecular orbital diagram for the π-molecular orbitals of butadiene as a result of combining the π-molecular orbitals of two ethene molecules. This shows the effect of conjugation.
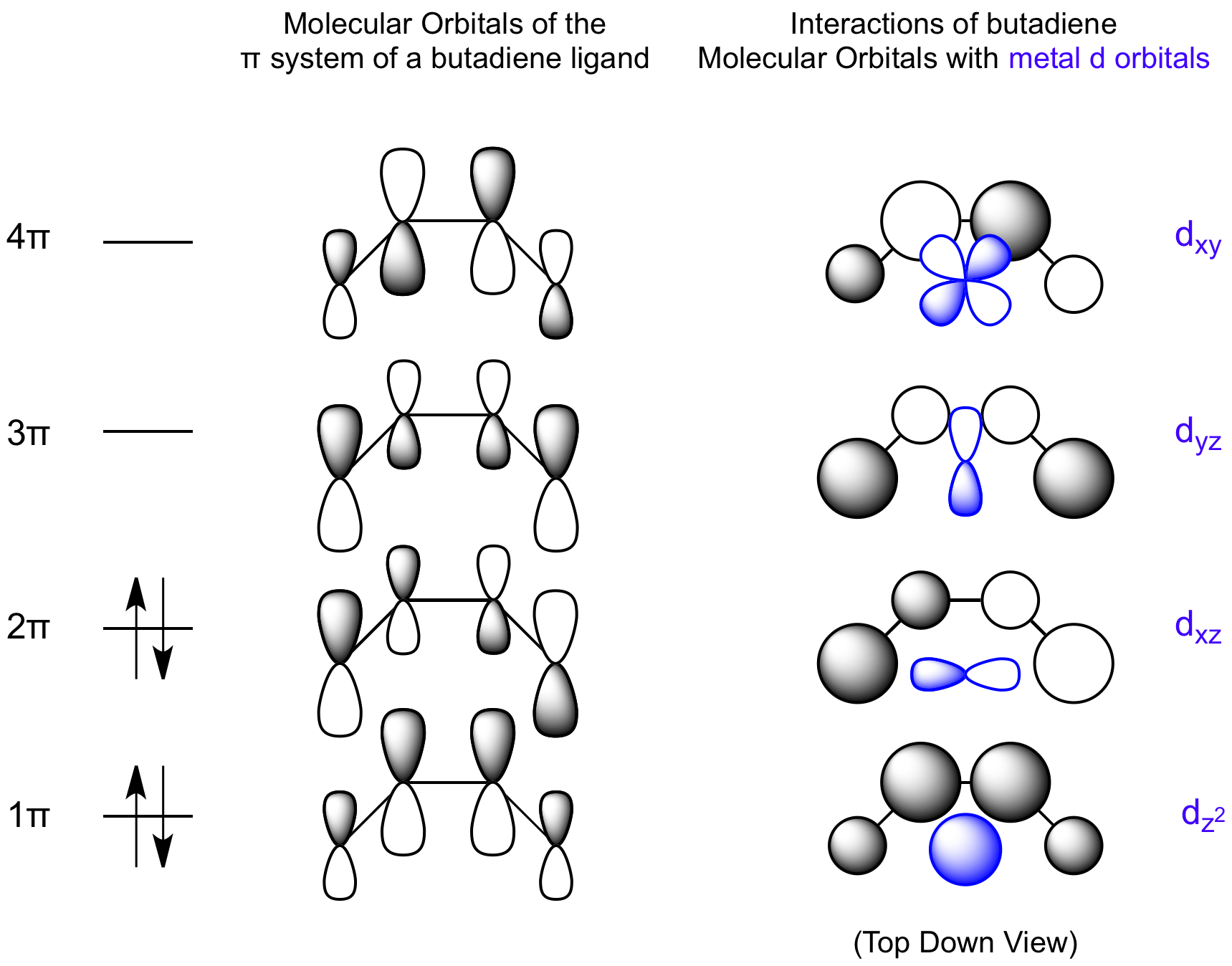
•The overall energy of the two bonding butadiene molecular orbitals is lower than that of the two molecular orbitals for ethene. A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals.
Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons. Bonding orbitals in Ethene (Ethylene) sp 2 Background: Use the buttons to display the sp 2 orbitals that make up the sigma framework and the remaining p orbitals which form the pi-bond.Molecular orbital theory: conjugation and aromaticity – Chemistry LibreTextsπ Molecular Orbitals of Conjugated Butadiene