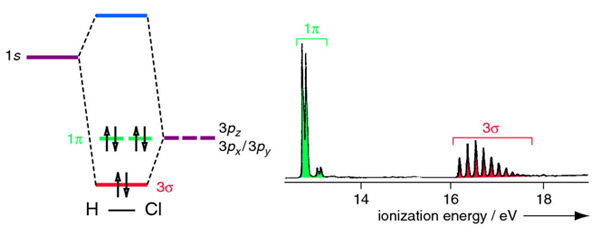
We shall consider the molecular orbitals in LiH, CH and HF to illustrate how molecular orbital theory describes the bonding in heteronuclear molecules, and to.
and 2p orbitals, but that is not how sodium chloride is made. Sodium atoms are Construct an MO diagram for LiH and suggest what type of bond it might have.
In this screencast, Andrew Burrows walks you through how to construct the MO energy level diagram of HF. basic idea behind molecular orbital theory – there are many variations on the bystep and deal with H2 and then Li2 and then LiH we will instead begin by.
We shall consider the molecular orbitals in LiH, CH and HF to illustrate how molecular orbital theory describes the bonding in heteronuclear molecules, and to.The molecular orbital energy level diagram of LiH in conventional textbooks for quantum chemistry is incorrect from viewpoint of ab initio Hartree-Fock SCF-MO calculation, because the 2σlevel of LiH is drawn at a lower position than the 1s orbital of H. It means that the 1s electron of H is stabilized by forming LiH. Describe the essential difference between a sigma and a pi molecular orbital.
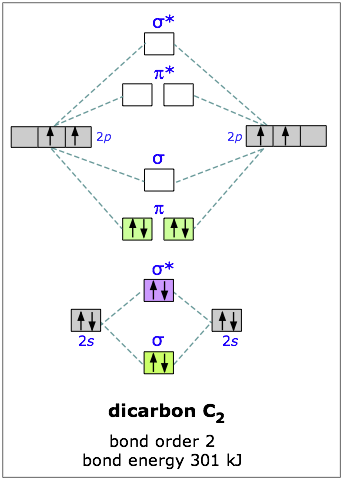
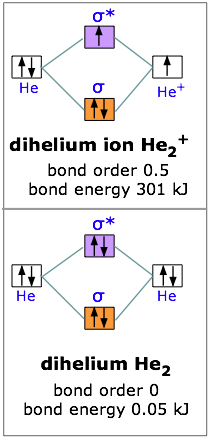
Define bond order, and state its significance. Construct a “molecular orbital diagram” of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable.
Aug 07, · Practice with the molecular orbitals of molecules by linear combinations of 2s and 2p orbitals. Remember, there is one 2s orbital and three p orbitals.

The orbital correlation diagram in predicts the same thing–two electrons fill a single bonding molecular orbital. To further demonstrate the consistency of the Lewis structures with M.O.
theory, we will formalize a definition of bond order–the number of bonds between atoms in a molecule. The 1s molecular orbital in LiH is to a good approximation a polarized doubly-occupied 1s orbital on Li, and the 2s molecular orbital is, to a somewhat poorer approximation, a .Molecular OrbitalsMolecular orbital diagram – Wikipedia