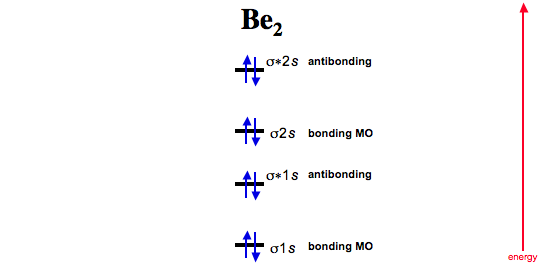
Molecular orbital theory: Energy level diagram Molecular orbital diagram of H2 + (Hydrogen molecule ion): Molecular orbital diagram of Li2 & Be2: Number. Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 .
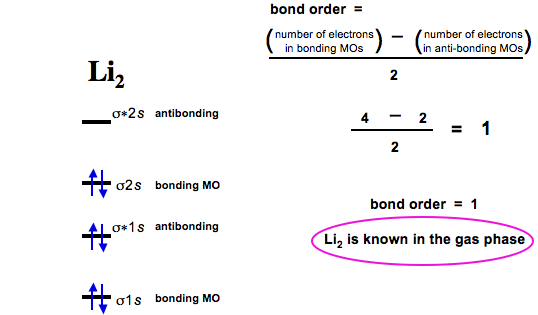
It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.
atoms in the molecule, whereas in molecular orbital theory, the electron pairs are . In our Li2 diagram, there are 2 electrons in bonding orbital at level 2, and 0.
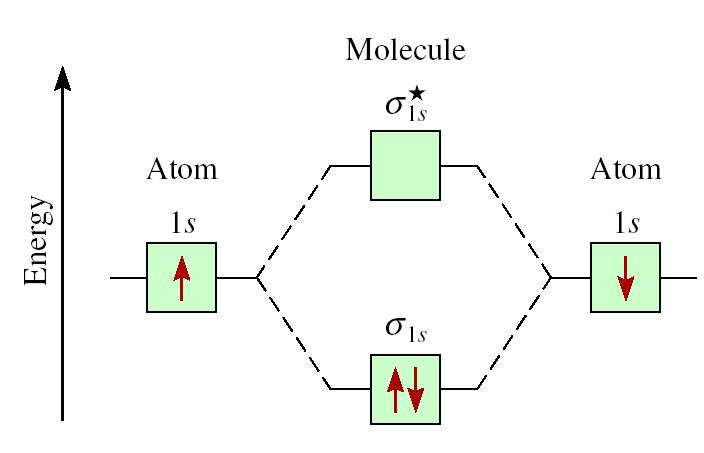
We represent this configuration by a molecular orbital energy diagram . electrons would be in a bonding orbital, we would predict the Li2 molecule to be stable.
Let’s follow these guidelines and generate a molecular orbital electron configuration diagram for Li2 (Figure “Molecular orbital electron configuration energy.3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy molecular orbital with respect to the 3s orbital of sulfur. This lowest energy orbital is essentially nonbonding.
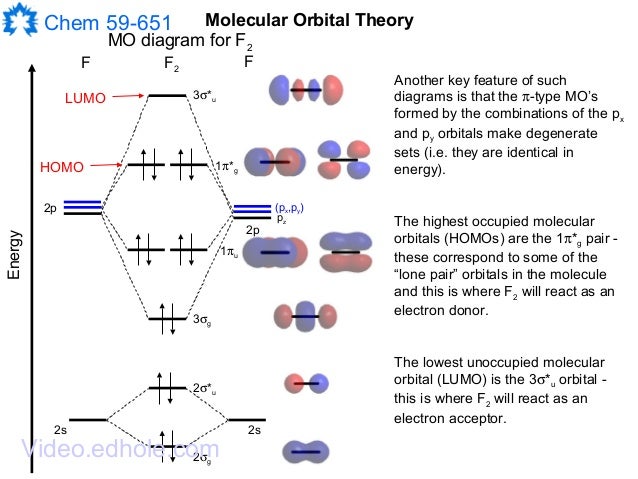
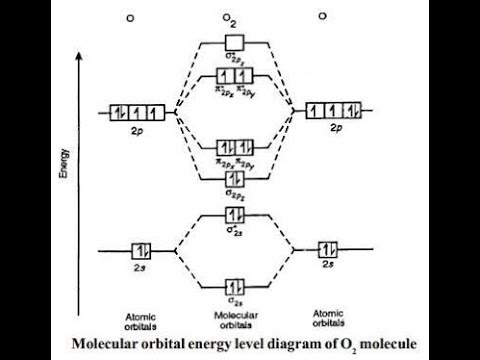
These orbitals are similar in appearance to those of HF in. Molecular orbitals: Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals of a molecule can be shown in a diagram called as molecular orbital diagram.
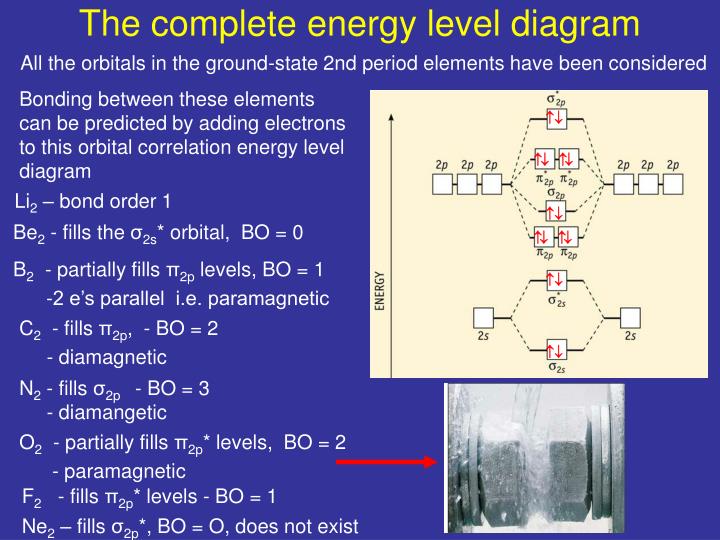
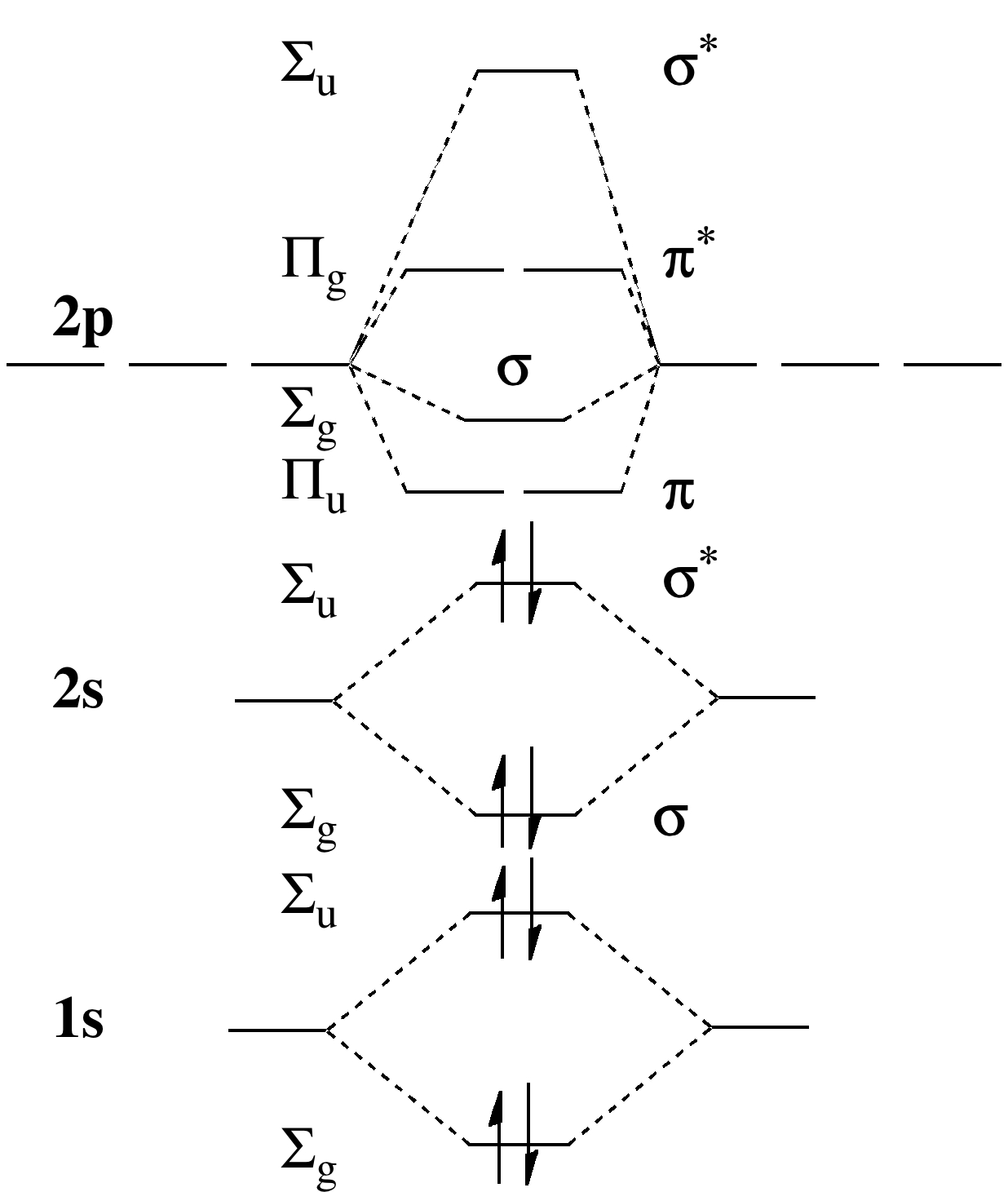
CAcT Home Molecular orbitals of Li 2, Be 2, to F 2 Skills to develop. Explain how the energy levels of atomic orbitals vary for H, Li, Be, B, C, N, and O.
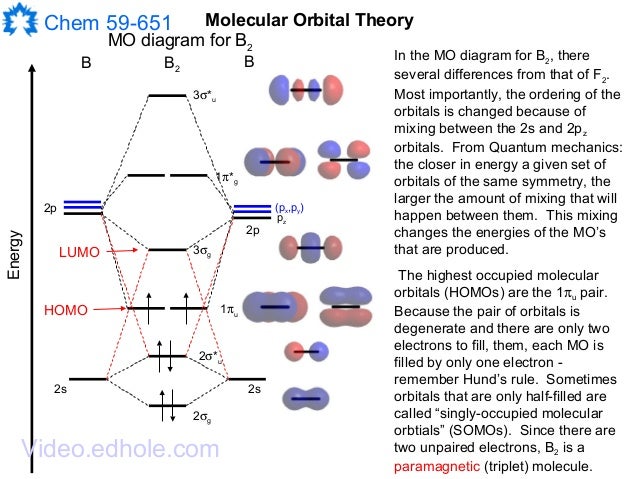
Draw relative energy levels diagrams for homonuclear diatomic molecules of period 2 elements. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before .
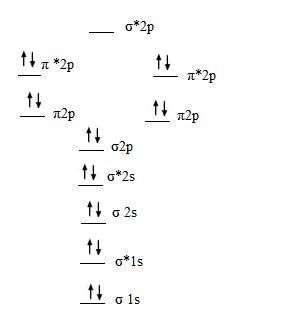
The molecular orbital theory of Li 2 to F 2 has given a diagramatic explanation of s p mixing leading to the difference in relative orbital energy levels. This link is from the University of Florida.
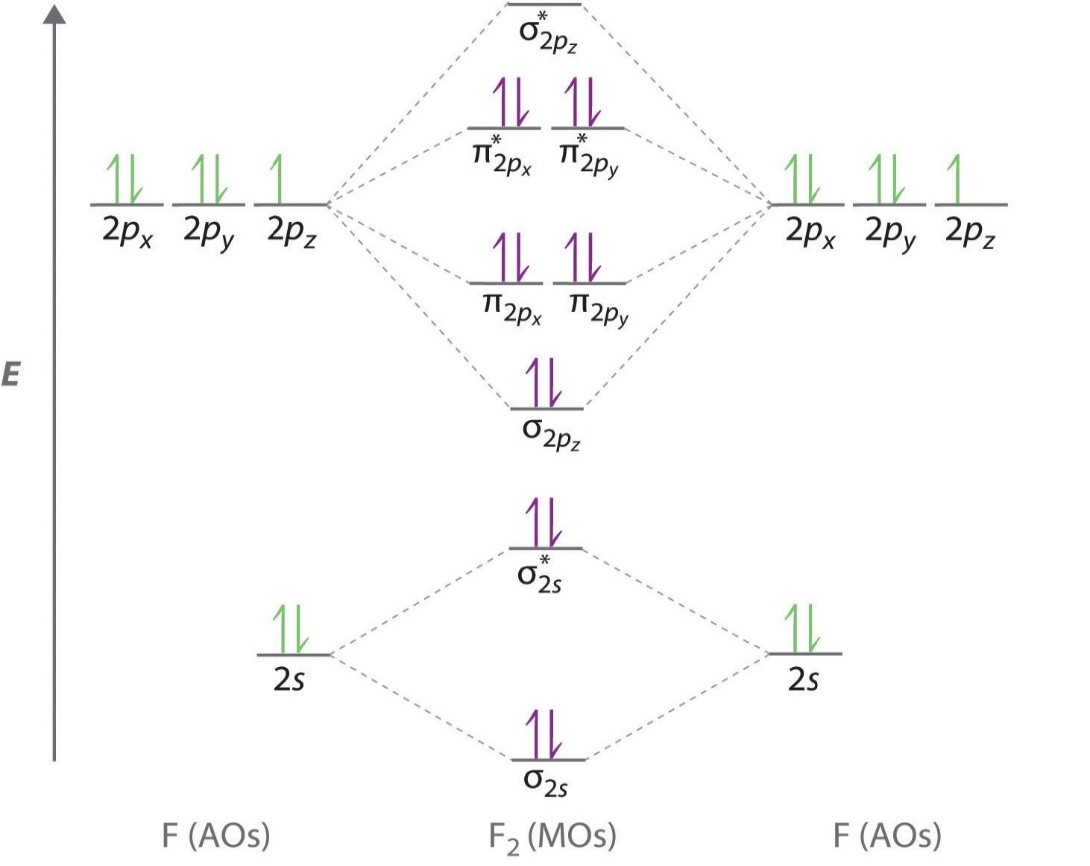
This link is from the University of Florida.Chapter 9, Section 8Molecular orbital diagram – Wikipedia