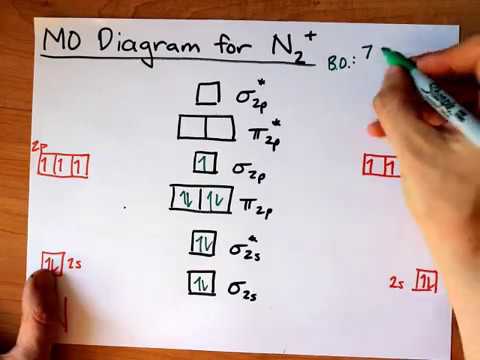
sorry about that not being a molecular orbital diagram i saw orbital and immediately thought electron configuration to save confusion, could.
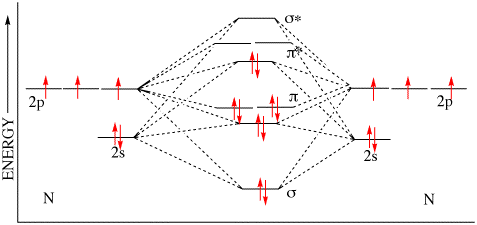
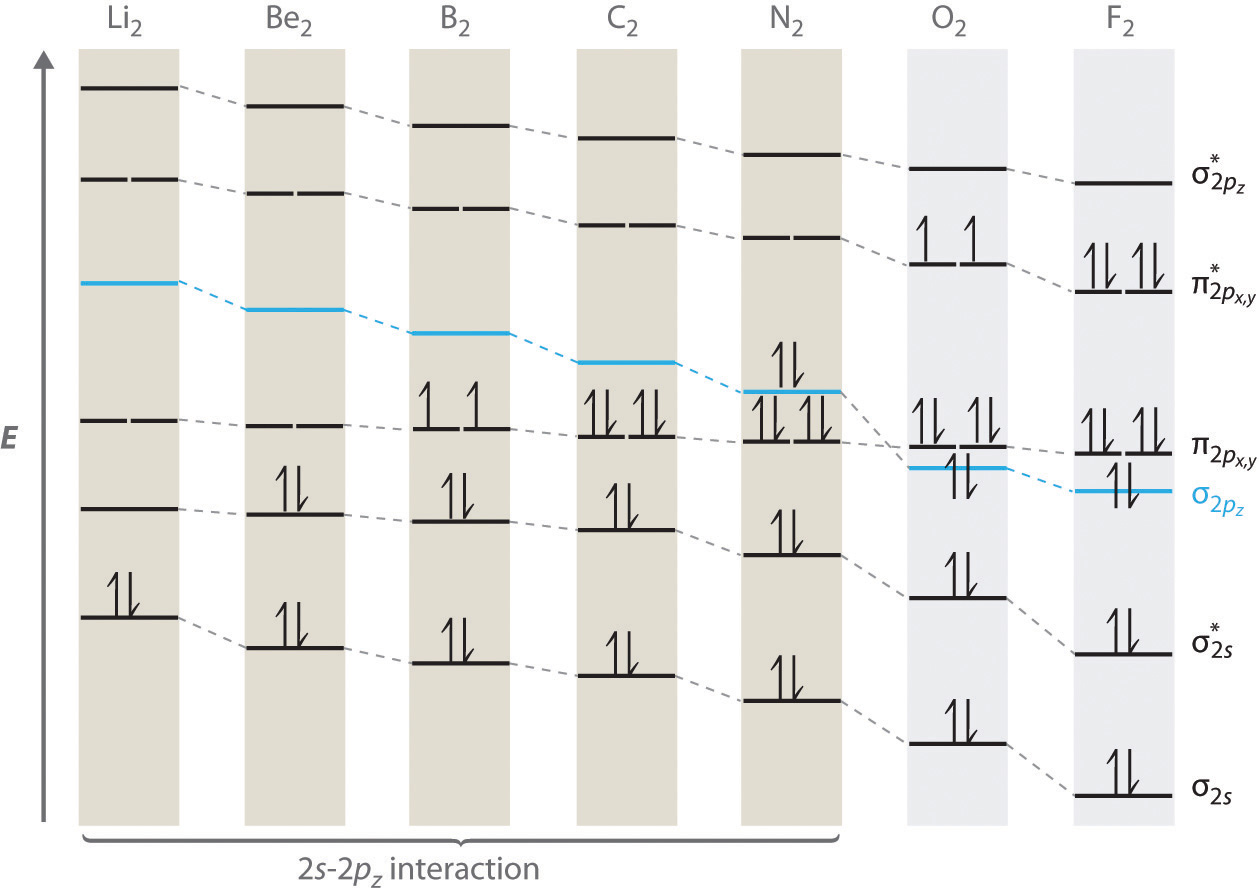
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc) . One is for the elements up to Nitrogen.
The other is for AFTER nitrogen. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves.

Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
This is because, according to molecular orbital theory , it has fewer electrons in bonding orbitals. The diagram above is the molecular.N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the energy than the atomic and form. MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules.

• Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals.
Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Molecular Orbital Theory – Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure.
In this case, the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital Theory – . Item 2: Part A Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below.M.O.
diagram for N2+Molecular orbital diagram – Wikipedia