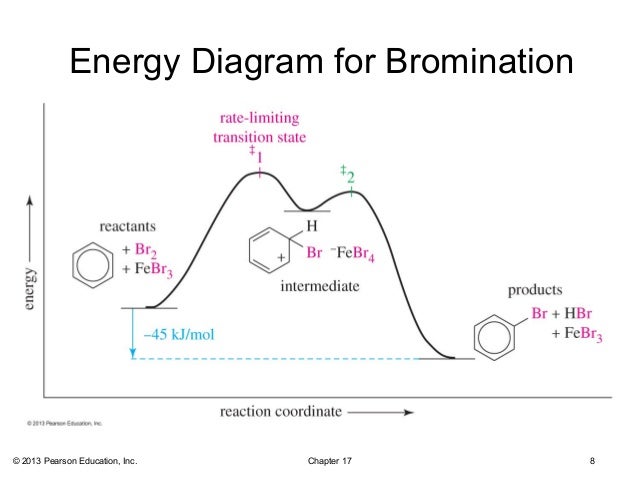
2) Please construct a potential energy diagram for the electrophilic aromatic substitution of benzene with an electrophile, E+.
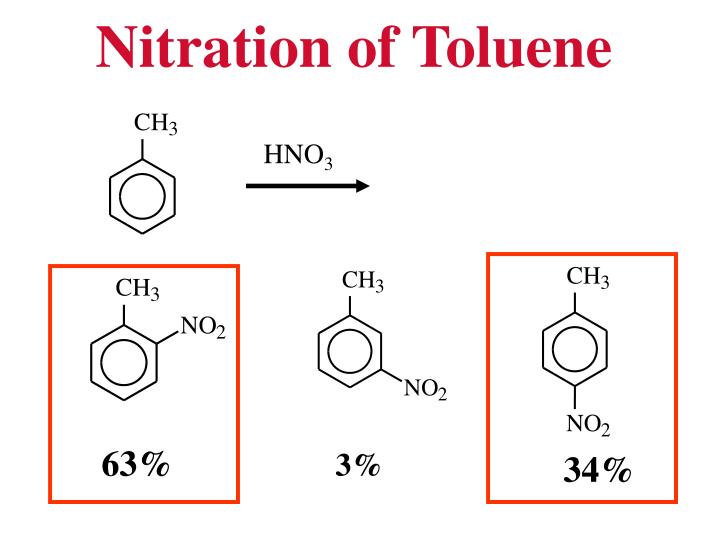
Your diagram should include. The Reaction Energy Diagram of Electrophilic Aromatic Substitution In the nitration of toluene, the product distribution is far from statistical.
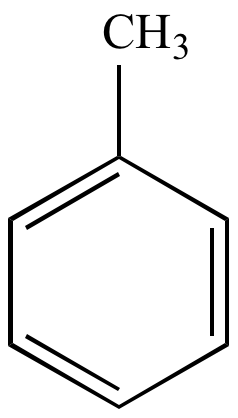
Chapter 7. Energy Diagram for Bromination Nitration of Toluene. ▫ Toluene reacts 25 times faster than benzene.
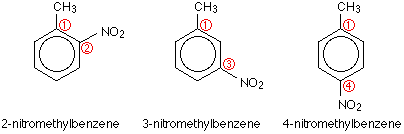
▫ The methyl group is an activator. The Reaction Energy Diagram of Electrophilic Aromatic Substitution In the nitration of toluene, the product distribution is far from statistical.

2) Please construct a potential energy diagram for the electrophilic aromatic substitution of benzene with an electrophile, E+. Your diagram should include.The methyl group of toluene makes it around 25 times more reactive than benzene in electrophilic aromatic substitution reactions.
Toluene undergoes nitration to give ortho and para nitrotoluene isomers, but if heated it can give dinitrotoluene and ultimately the explosive trinitrotoluene (TNT). of toluene diisocyanate (TDI), including nitration of toluene to produce dinitrotoluene (DNT), hydrogenation of DNT to produce toluene diamine (TDA), production of phosgene from c arbon monoxide and chlorine, gas-phase phosgenation of TDA to .
3. Why is it important that the nitration of benzene by nitric acid occurs in sulfuric acid? 4.

Write a detailed mechanism for the sulfonation of benzene, including all resonance forms. 5.
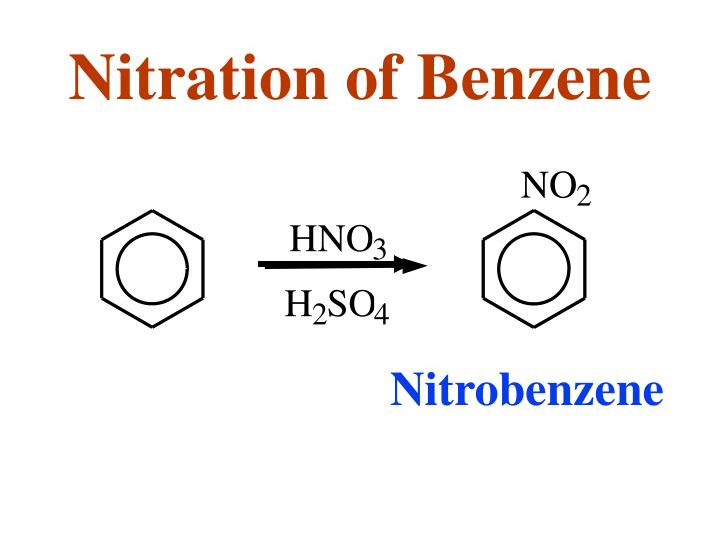
Draw an energy diagram for the nitration of benzene. Draw the intermediates, starting materials, and .
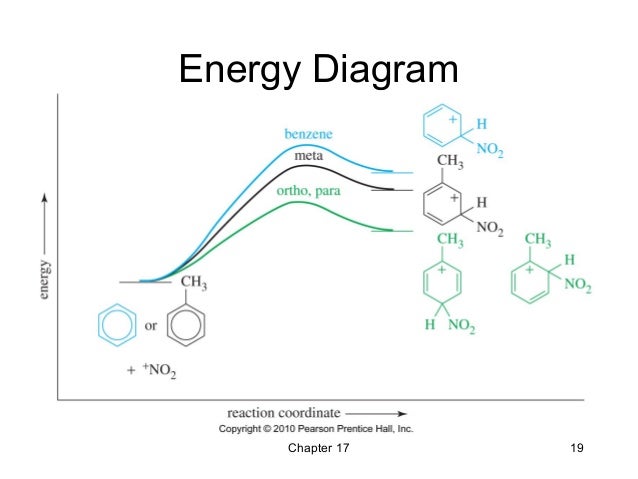
Describe the energy diagram of chlorine deactivating relative to benzene. Benzene has lower energy and reacts faster than meta, para, and ortho.
However, para and ortho are lower than meta, so the nitration of chlorine is ortho/para deactivator. The structure on the right has two benzene rings which share a common double bond. From heats of hydrogenation or combustion, the resonance energy of naphthalene is calculated to be 61 kcal/mole, 11 kcal/mole less than that of two benzene rings (2 * 36).Chapter 12 – Reactions of Benzene – EAS – ppt video online downloadEnergy Diagram for Nitration of Toluene