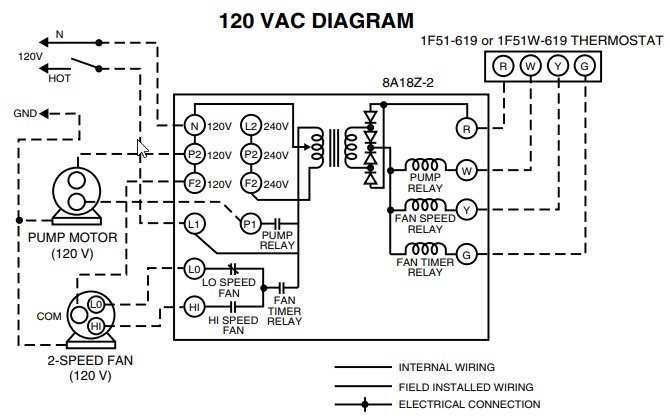
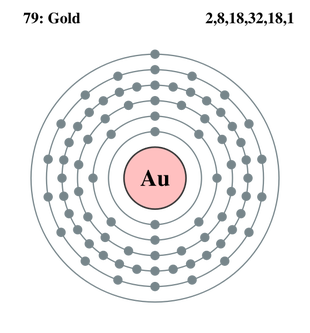
What is the electron configuration of Au+?
The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Orbital Diagram. 1s.
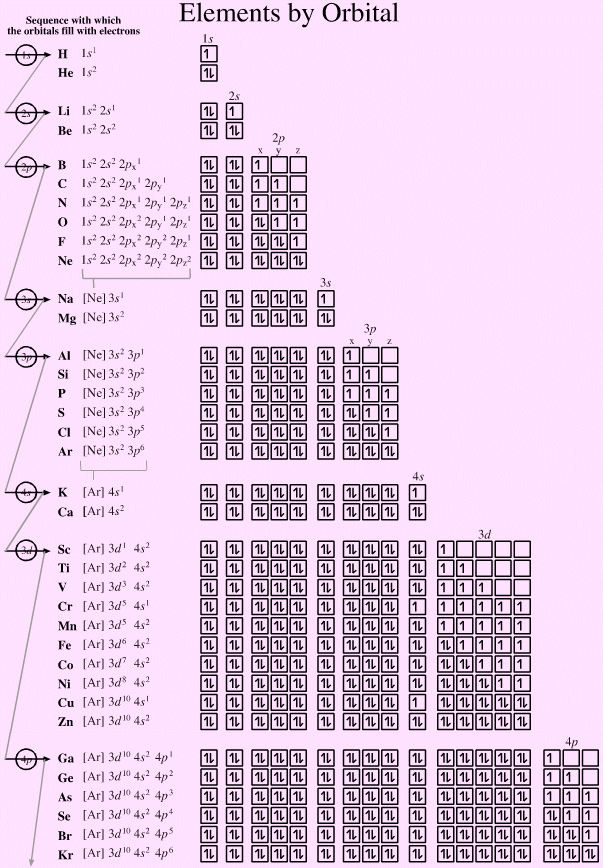
↿⇂. 2s. ↿⇂. 2p.
Write orbital diagram for Au+?
↿⇂. ↿⇂. ↿⇂.
Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1.
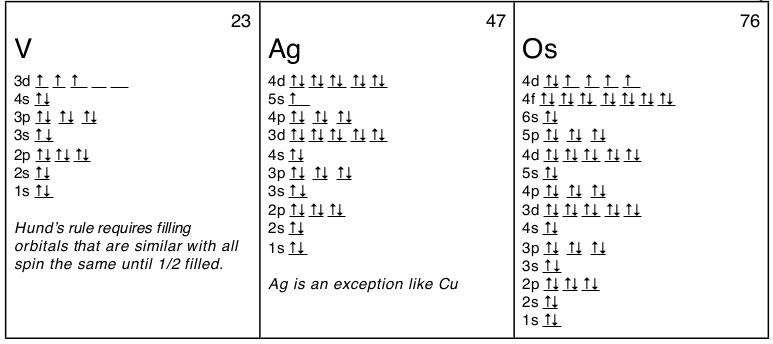
Orbital Diagram. 1s.
What is the electron configuration of Au+?
↿⇂. 2s. ↿⇂.
2p. ↿⇂.
↿⇂. ↿⇂. the MO diagram for AuOА and AuSА and Fig.
3 shows the. MO pictures for AuOА. The O px/y orbitals and Au 5dxz/yz orbitals form the bonding.
Similar Questions. Chemistry.
Write orbital diagram for Au+?
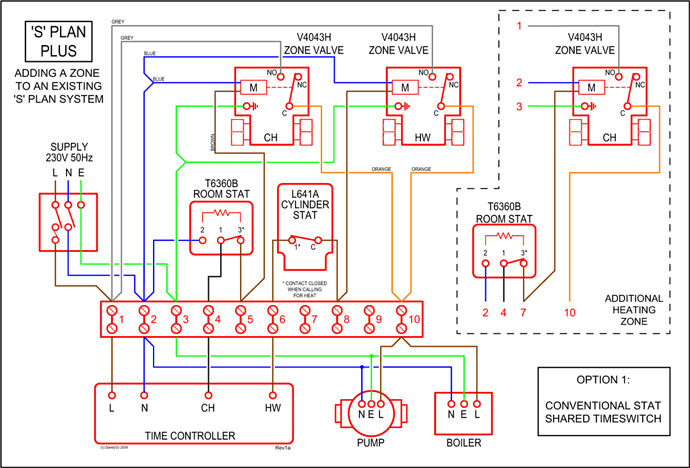
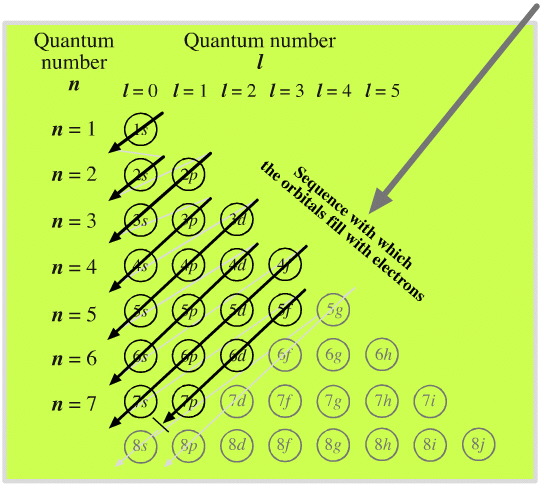
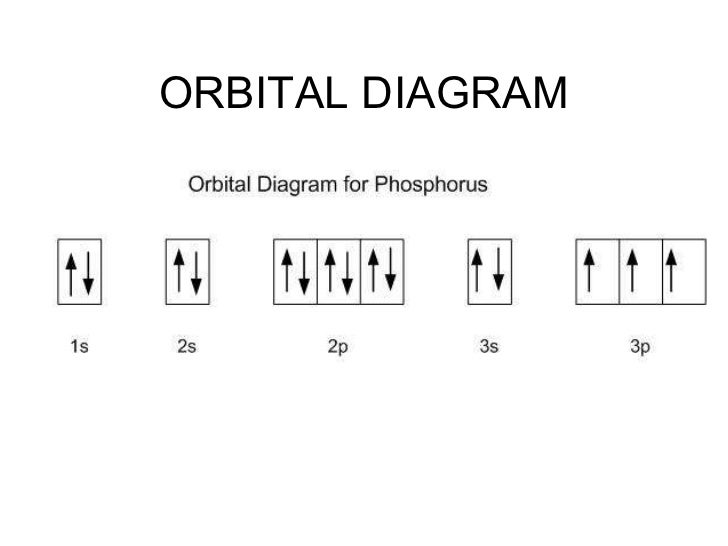
(1) Which of the following clusters of orbitals would form the shape trigonal bipyramidal and would also be possible within the.Write the orbital diagram for Au+ Get Answer. Recently Asked Questions Five thousand bonds with a face value of $ each, are sold at The entry to record the issuance is a-Cash Discount on Bonds; Please refer to the attachment to answer this question. . 3 SAMPLE PROBLEM Determining Quantum Numbers from Orbital Diagrams PLAN: SOLUTION: Use the orbital diagram to find the third and eighth electrons.
PROBLEM: Write a set of quantum numbers for the third electron and a set for the eighth electron of the F atom. Table Partial Orbital Diagrams and Electron Configurations * for the Elements in Period 4.
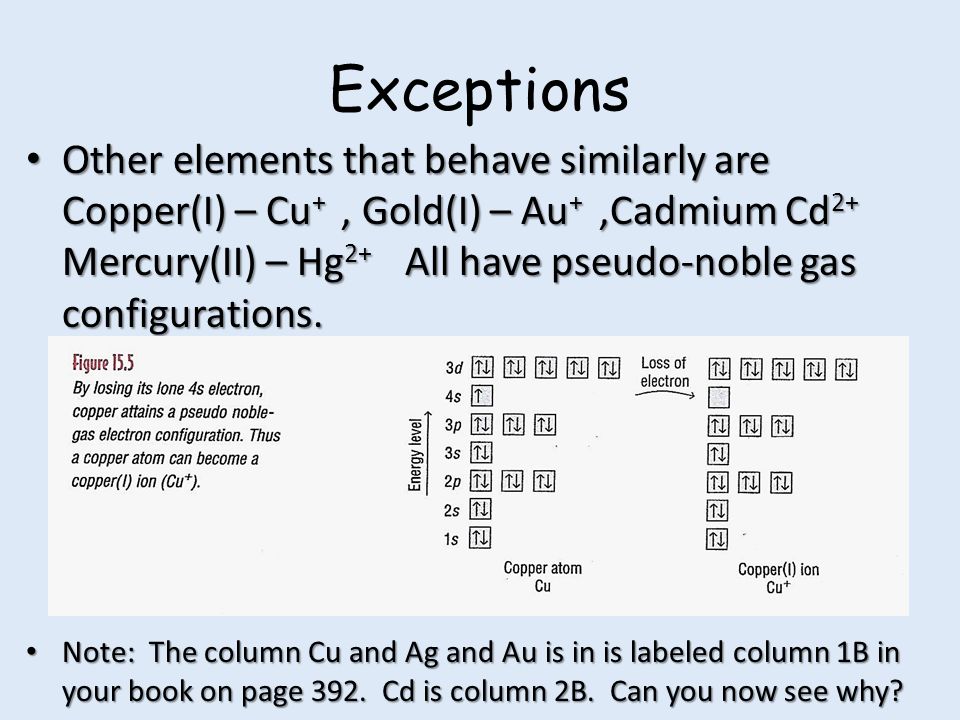
* Colored type indicates the sublevel to which the last electron is added. Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+.
Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy.

Click within the orbital to add electrons. Jul 21, · Write orbital diagram for Au+ Determine if the ion is diamagnetic or paramagnetic. please helpStatus: Resolved.what is the orbital diagram for Au+, how do you fit the f orbitals in?what is the orbital diagram for Au , how do you fit the f orbitals in?