Step by Step: Electron Configurations and Electron Orbital Diagrams we need to count each box going from Hydrogen (#1) to Barium (#56), including Barium.
The first two groups (columns) of the periodic table represent the ‘s’ orbital group. This means that the s,p,d,f electron configuration for Barium.
Barium, complete electron configuration. Barium.
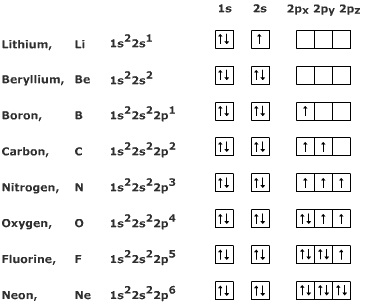
Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. Diagram of the nuclear composition and electron configuration of an atom of barium (atomic number: 56), the most common isotope of this element.
Barium (BA 56)
Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium (atomic number: 56), an isotope of this .Barium is an alkaline earth metal. This means that it is a group 2 element.
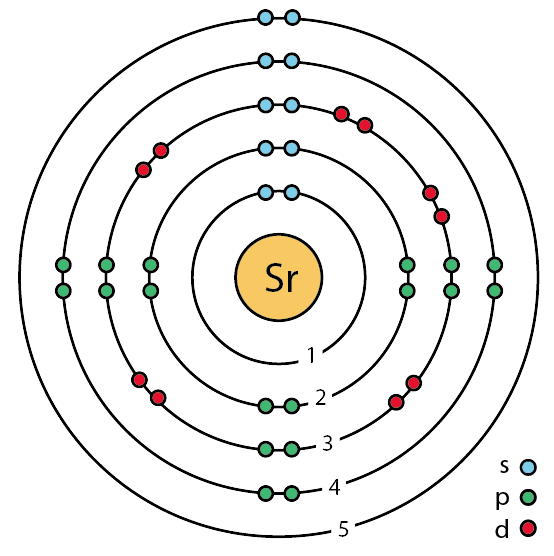
Barium’s atomic number is 56; this means that it has 56 protons in its nucleus and also puts it as a period 6 element. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital.
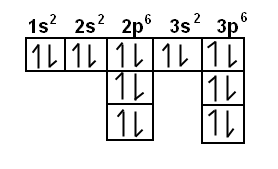
Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Barite, or barium sulfate (BaSO4), when ground is used as a filter for rubber, plastics, and resins.
It is insoluable in water and so is used in X-rays of the digestive system. Barium nitrate, Ba(NO3)2, burns brilliant green and is used in fireworks.

Barium can be found in the 6th energy level (row) of the periodic table. It is also in the 2nd group (column) of the periodic table.
The first two groups (columns) of the periodic table represent the ‘s’ orbital group. This means that the s,p,d,f electron configuration for Barium must end with 6s^2. The 6th row, s block, 2nd column.
May 15, · what are the bond angles in BaSO4? teh shape of the bonds teh type of compound the Ksp expression and value: The Keq expression: all of that is for Barium sulfate. i have a huge chemistry project due tomorrow and i have done all that i can figure out.
and im not doing this last second i just havent had a computer except durring school for 1 hour for the last 5 days.. so i need your help!Status: Resolved.Electron Configuration | Wyzant ResourcesBohr model – Wikipedia