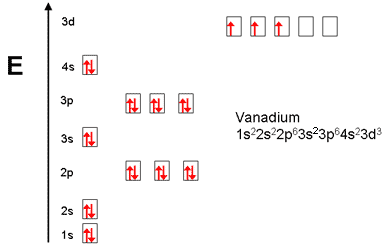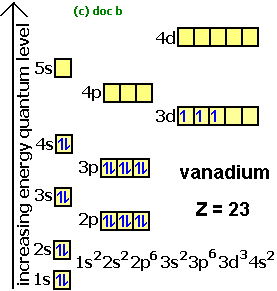
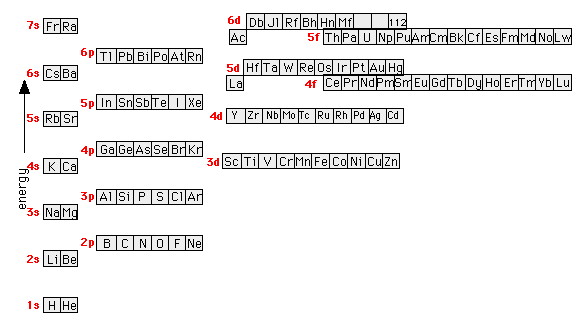
orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four .
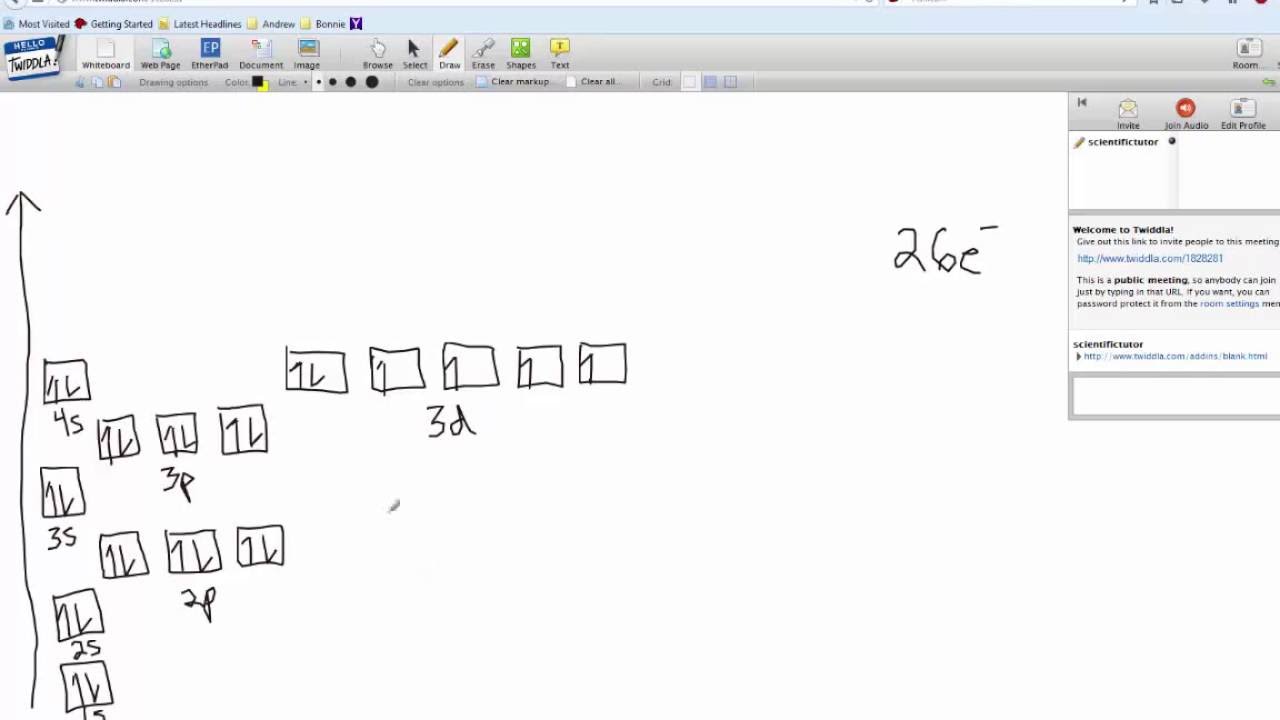
The orbital diagram for germanium is. 1s. 2s.

2p. 3s. Oxidation States, +4,2.
Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
Orbital Diagram. 1s.
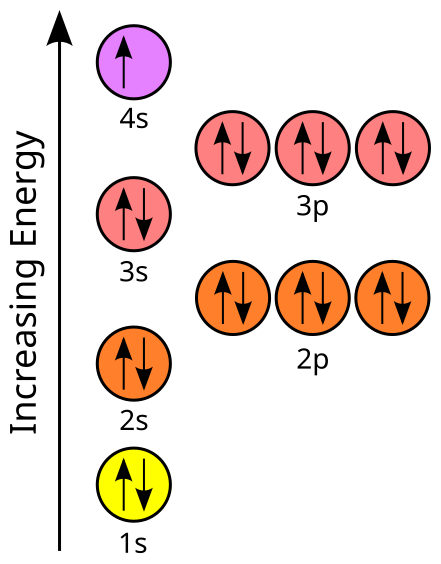
↿⇂. 2s.
Comprehensive data on the chemical element Germanium is provided on this page; including scores of properties, element names in many languages, most. Use it to learn, and these orbital diagrams won’t be a problem!
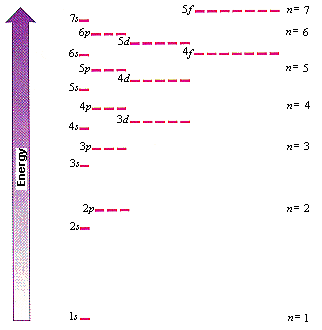
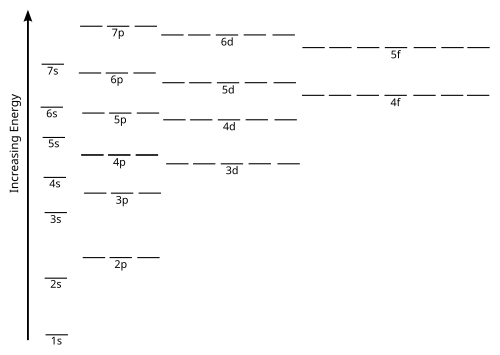
how do you draw the orbital diagram for an atom of germanium (Z = 32)?. Mendeleev’s Predicted Properties of Germanium (“eka. Silicon”) and Its Use the orbital diagram to find the third and eighth electrons.
PROBLEM: Write a set of .Which ground-state atom has an electron configuration described by the following orbital diagram? A antimony B germanium C indium D lead E tin%(1).
Scitation is home to the most influential news, comment, analysis and research in the Physical Sciences. When drawing the orbital diagram for an element, the elements up to the noble gas configuration are not necessary. As such, which represents the BEST selection of orbitals to draw an orbital diagram for Molybdenum?
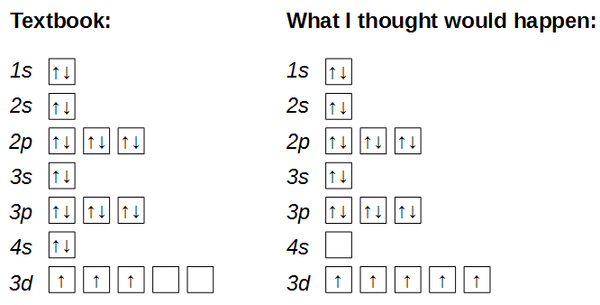
A. 4s and 4d B. 4s, 4d, and 4p C.

5s, 5d, and 5p D. 5s and 4d E.
. Introduction.
There are two types of semiconductor components in electronic and electrical circuits. They are active and passive components.
Diodes are the foremost active components and resistors are the foremost passive components in electronic design circuits. B) f orbital Basically, when l = 0 the is one orbital and it is called an s-orbital.
It can hold 2 electrons total. When l = 1, the orbitals are called p-orbitals and there are 3 of them.
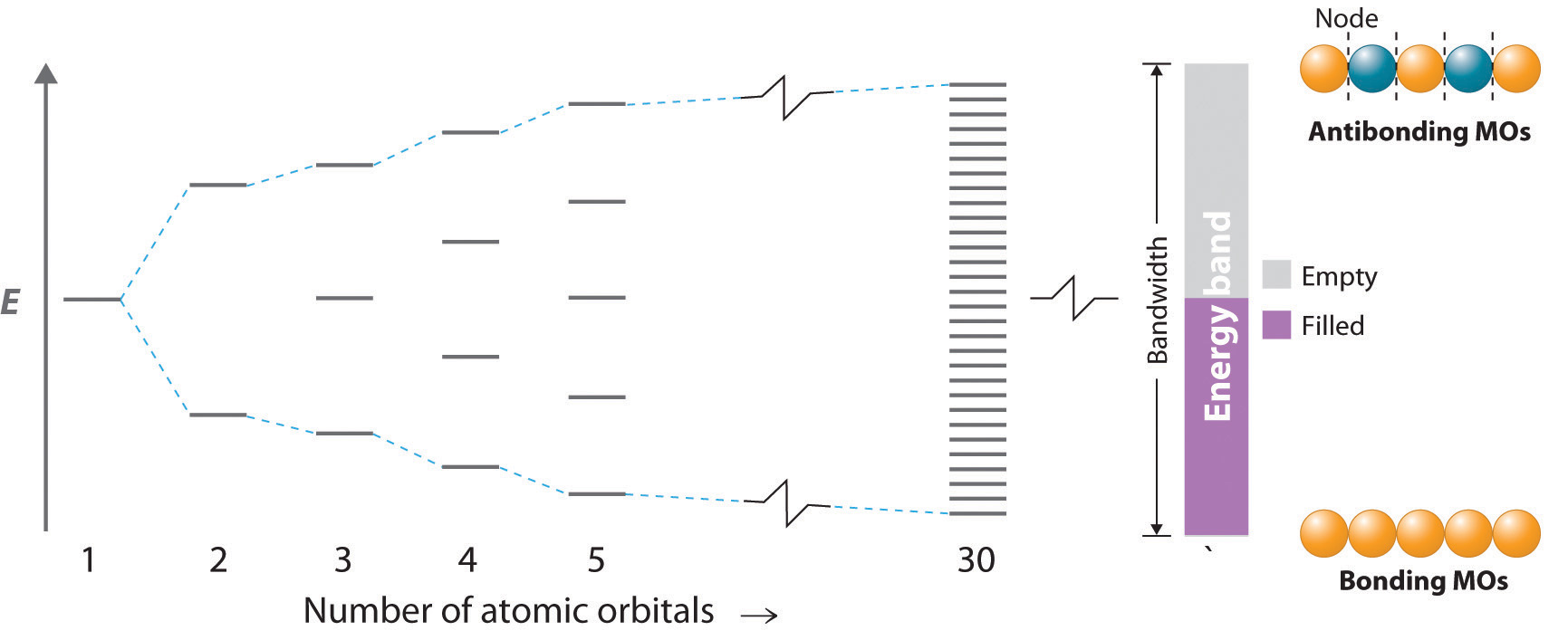
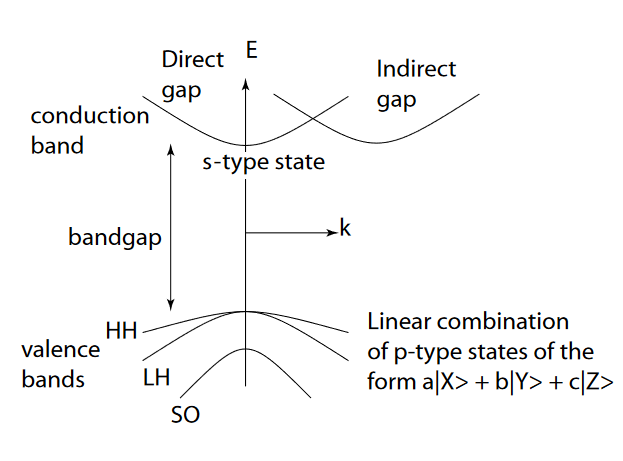
Each of the individual p-orbitals can hold 2 electrons each. This gives us a total of 6 electrons that can go into the 3 p-orbitals.Bonding in Metals and SemiconductorsThe oxides of carbon, silicon, germanium, tin and lead