
In general, electrons are removed from the valence-shell s-orbitals before they are removed from valence d-orbitals when transition metals are.
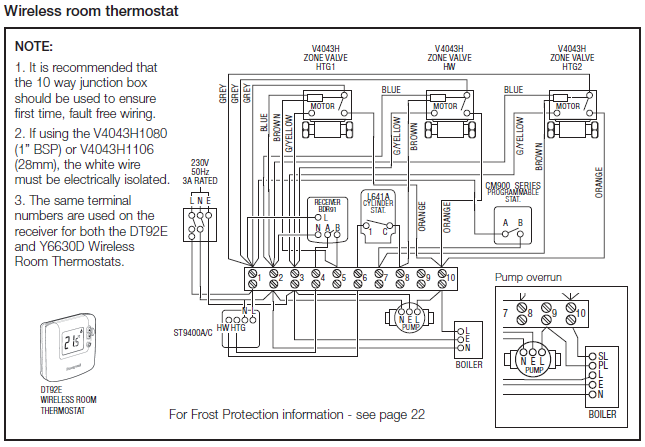
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V.
Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are.
Drawing electron configuration diagrams | Chemistry for All | The Fuse Electron Configuration For Vanadium – V, V2+, V3+, and V5+ Ions Quantum Numbers Explained – Electron Configuration, Atomic Orbital Diagrams – S P D F & n l ml ms . Pourbaix diagrams are useful in predicting the stabilities of various species.
A discussion of these diagrams can be found in schematron.org Educ. , 46, Nov 06, · This video shows you how to write the electron configuration for the vanadium atom and many of its ions – V, V2+, V3+, and V5+.
So let’s look at the Auf Bau diagram which actually show this for us, okay so down here we have the 1s orbital but the 1 dash indicates that there’s 1 orbital within the 1s sublevel which makes sense that it is the lowest in energy, it’s its first principle energy level. Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic.
V 86%(14). Write orbital diagrams for each of these ions. A.
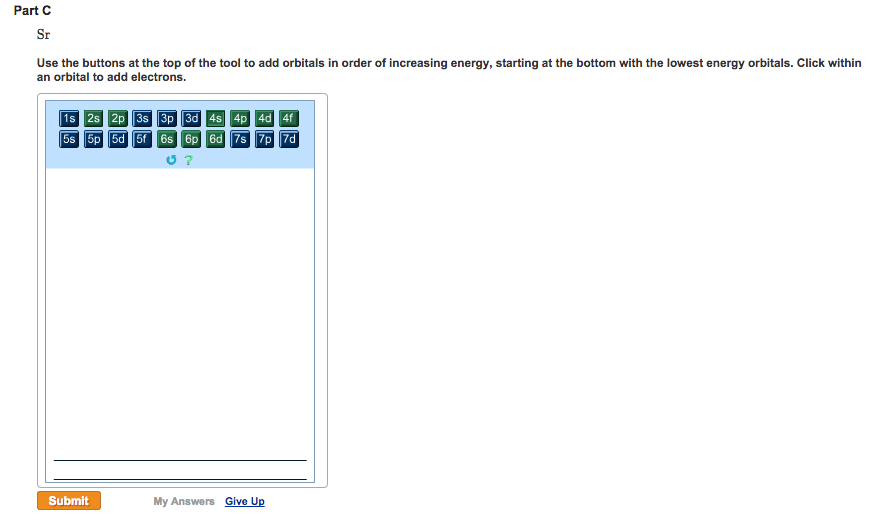
V^5+ B. Cr^3+ C.
Ni^2+ D. Fe^3+ E.
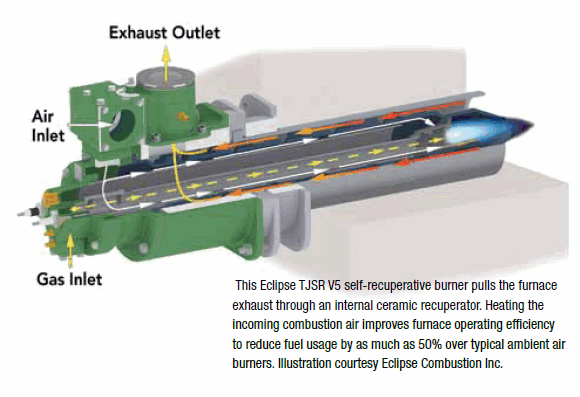
Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system lists the list of keywords related and the list of websites with related content, in addition you can see which keywords most interested customers on .inorganic chemistry – Why is the vanadium(3+) ion paramagnetic?
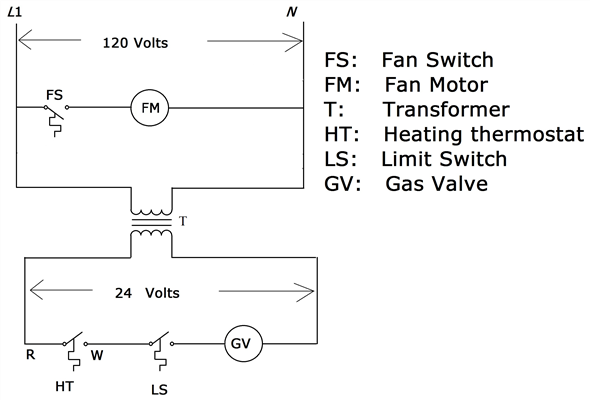
– Chemistry Stack ExchangeMolecular orbital diagram – Wikipedia