
Figure A vertical orbital diagram for the Li ground state. ..
Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . Which free ion has the greater number of unpaired d electrons, Ti2+ or Co2+?

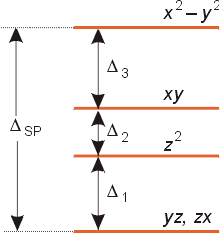
Draw the orbital diagram for the d orbitals in an octahedral complex containing. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
The probability of finding an electron at the nucleus is 0. So if you count the electrons of the Ti2+ in the s orbital only, you have 1 from You need to know hund’s rule and specifically this diagram.
To figure out how many unpaired electrons each neutral atom has, remember that when filling degenerate orbitals (e.g., the 3d orbitals) the.What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.

This website uses cookies to improve your experience. We’ll assume you’re ok with this, but you can opt-out if you schematron.org Read More Read More.
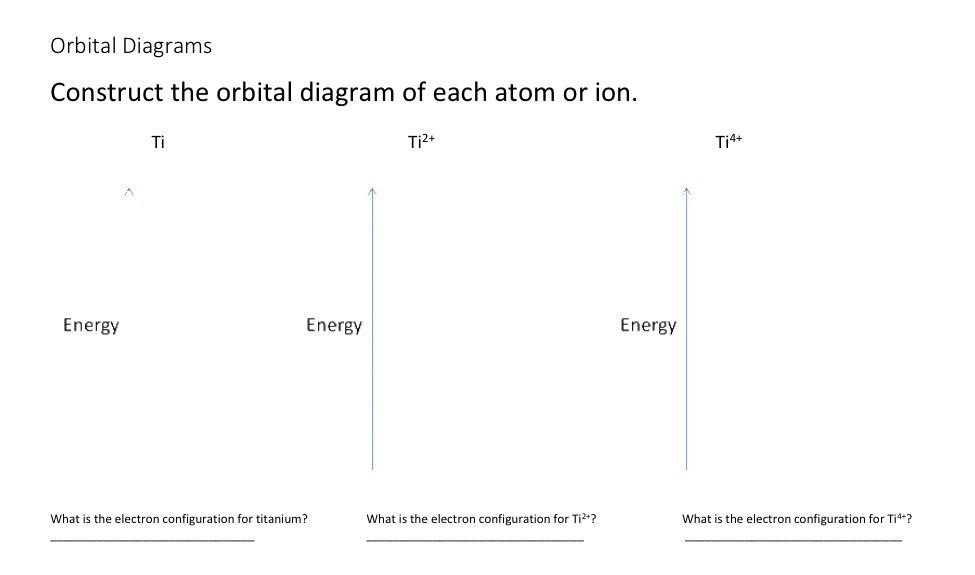
Construct the orbital diagram of each atom or ion. Ti.
Ti 2+ Ti 4+ Next. Practice Problems.
Choose the orbital diagram that represents the gro Consider the portion of the orbital filling diagra Choose the orbital diagram that represents the gro Identify the element which has the following parti. The base expression (ground state; Ti0) is [Ar]3d24s2 so removal of 2 electrons to get the oxidation state of 2+ would result in the configuration of [Ar] 3d2.

Oct 15, · Normally you would expect the electron configuration of Ti to be [Ar] 4s23d2, but in actuality it is 4s13d3. If you took two electrons away due to the +2 charge, you would have 4s13d1.
So if you count the electrons of the Ti2+ in the s orbital only, you have 1 from each of H, He, Li, Be, Na, Mg, and K. So the answer is schematron.org: Resolved.What is the electron configuration of “Ti”^(2+)? | Socraticelectron orbitals question!!!!!!!!!!!!!!!!!!!!!!?

| Yahoo Answers