
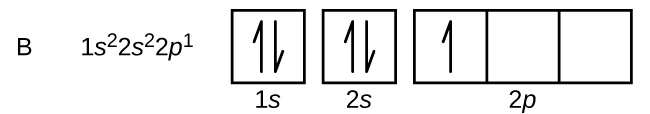
Boron is the fifth element with a total of 5 electrons.
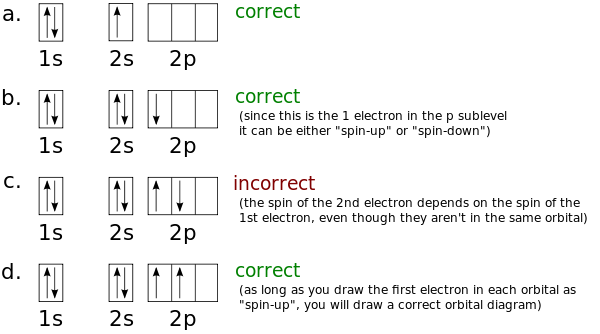
In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down.
1s2, 2s2, 2p1 Boron 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d1. Scandium.
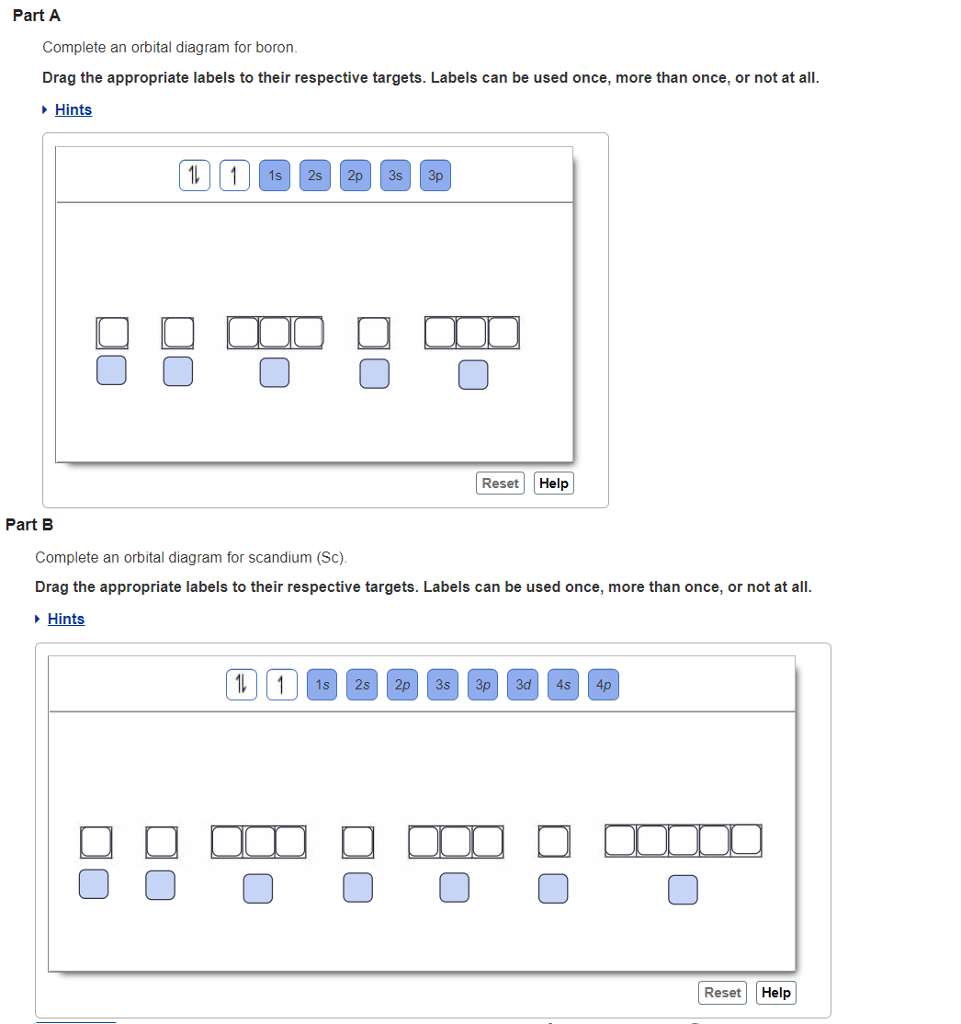
Answer to Draw an orbital diagram for boron. Use this tool to draw the orbital diagram.
Draw an orbital diagram for scandium (Sc). From the orbital diagram, we can write the electron configuration in an abbreviated When we reach boron, with Z = 5 and five electrons, we must place the fifth .
After filling the first five rows, we still have 80 − 54 = 26 more.Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.
In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Show transcribed image text Draw an orbital diagram for boron.
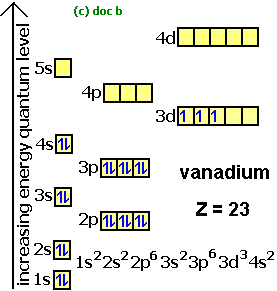
Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc).
Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)?
Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen)%(8).
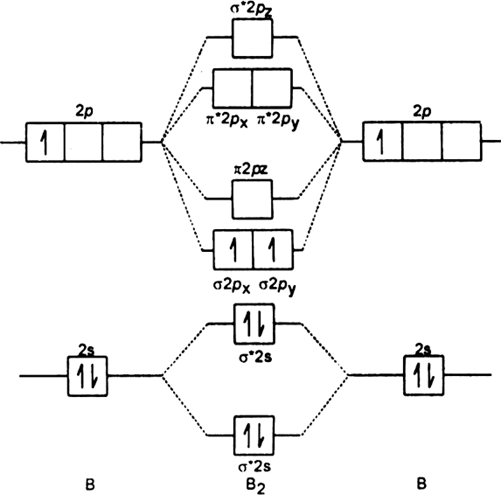
The outer-most electrons are the only ones included in the orbital filling diagram and the electron dot diagram because the outer-most electrons are the only ones that need to be used in chemical reactions and bonding, so the other electrons are insignificant in these diagrams. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital.

The remaining electron will go in the 2p orbital.Hund’s Rule and Orbital Filling Diagrams – Chemistry LibreTextsMolecular orbital diagram – Wikipedia