Oxidation States, ±1,+5.

Electrons Per Shell, 2 8 18 7. Electron Configuration, [ Ar] 4s2 3d10 4p5.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Orbital Diagram. 1s.
↿⇂. This mini lecture is about using th Aufbau principle on Bromine. Answer to Show the orbital-filling diagram for (bromine).
Stack the subshells in order of energy, with the lowest-energy sub shell. Note: + represent arrow going up, – represent arrow going down, and ___ represent the number of orbitals in sub shell.
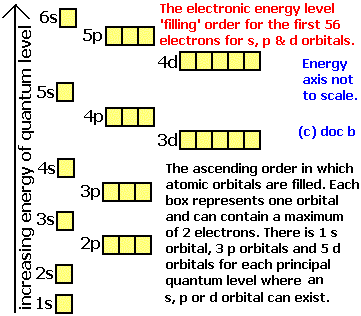
notice diagram is off. There are 3 main rules for filling atomic orbitals.

1. When filling a sublevel with multiple orbitals (p, d, or f), each orbital gets one Orbital Diagram for Bromine.Show the orbital-filling diagram for S (sulfur).
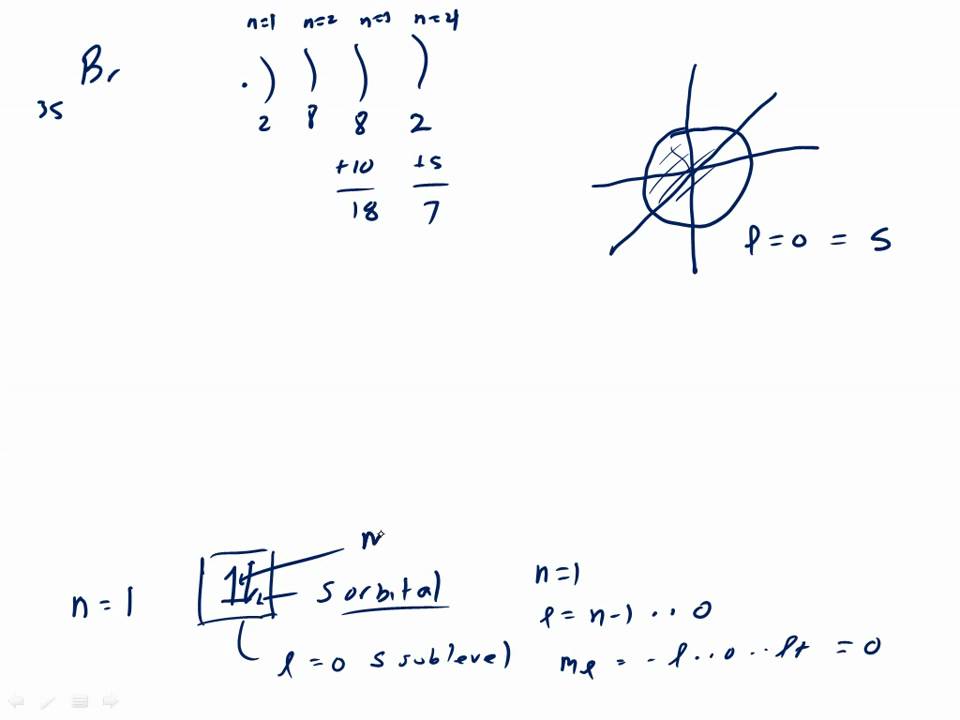
Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for Br (bromine). Orbital Notation is a way to show how many electrons are in anorbital for a given element.
They can either be shown with arrowsor circles. One arrow represents one electron in a shell. Twoarrows will be pointing differently; one up and one down to show amaximum of two electrons with different spin.
The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Orbital filling diagram Atomic orbital diagram for bromine frompo.
See extra concepts approximately Orbital filling diagram from Diagram. Atomic orbital diagram for bromine frompo gallery & create your own home design.
Orbital filling diagram is in reality amongst photographs libraries inside of our very best pictures gallery. Describe the two differences between a 2p x orbital and a 3p y orbital.

The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital.High School Chemistry/Electron Configurations – Wikibooks, open books for an open worldOrbital notation for bromine