Orbital diagrams
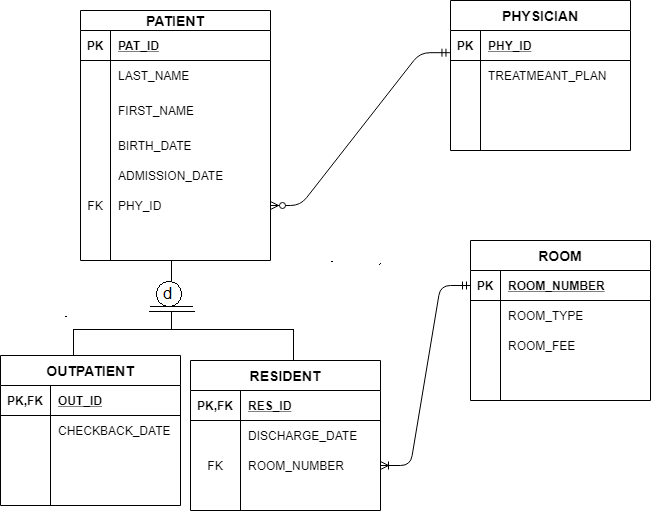
Orbital diagrams are pictorial representations of the arrangement of Rather than filling the orbitals one-by-one, it is easier to note that calcium is in the s- block. When we write the configuration we’ll put all 20 electrons in orbitals around the nucleus of the Calcium atom.
Here I use the electron. Write the electron configuration and draw an orbital diagram, showing the Calcium.

1s2 2s2 2p6 3s2 3p6 4s2. 3.
Iron. 1s2 2s2 2p6 3s2 3p6 4s2 3d6. 4.
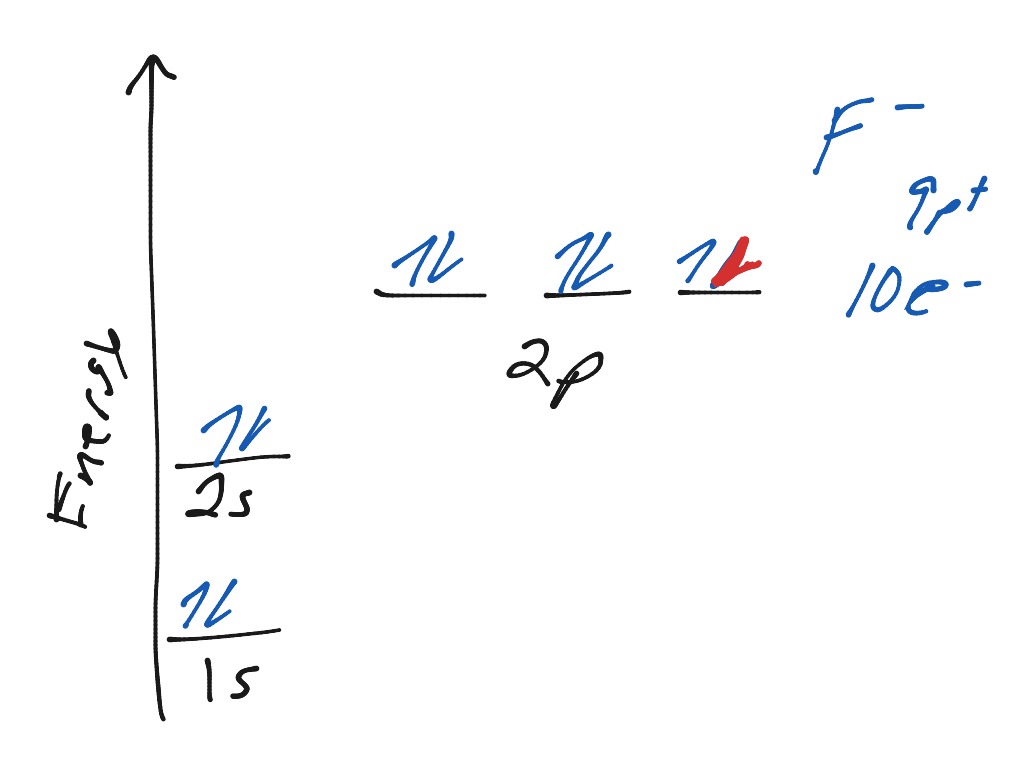
Do u mean a Bohr diagram? Where you draw the nucleaus and they you draw the surrounding rings and fill them with the correct number of. In order to write the Calcium electron configuration we first need to know the we’ll put all 20 electrons in orbitals around the nucleus of the Calcium atom.Mar 26, · This video tutorial shows you how to fill the orbital diagrams using boxes and arrows plus how to write the electron configuration for the elements Calcium (Ca) and Chlorine (Cl).
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital.
Atomic Orbital Diagram of Calcium (2+) Ion
Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
How to Write the Electron Configuration for Calcium (Ca)
Electron Configurations and Orbital Diagrams KEY Draw orbital diagrams for the following elements: 1. phosphorus ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑ ↑ ↑ 1s 2s 2p 3s 3p 4s 3d 4p.
Okay let’s do the orbital diagram for iron, iron we know is on its ground state of 26 electrons, so we know the first electrons are going to go into the 1s orbital and we said 2 electrons can fall into the 1s orbital. The orbital filling diagram of lithium.
The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital.Electron Configuration for Calcium (Ca)What is the orbital notation of calcium