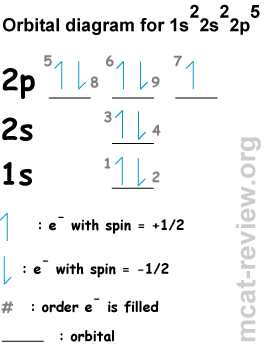
In sulfur trioxide the bonding electrons are filling the molecular orbital, not the atomic orbital such as 4s or 3d. It is forbidden that both 3s and 4s.
Answer to Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell.
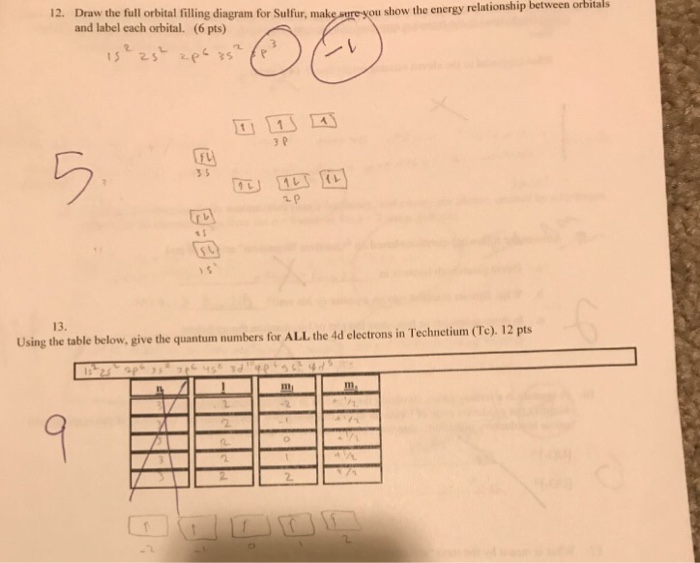
Learning and teaching resource for Atomic Orbitals and Electron Configurations written by This is just to help orient you when filling out the energy level diagram. First, let’s go to the periodic table and find how many electrons sulfur has. Show the orbital-filling diagram for S (sulfur).
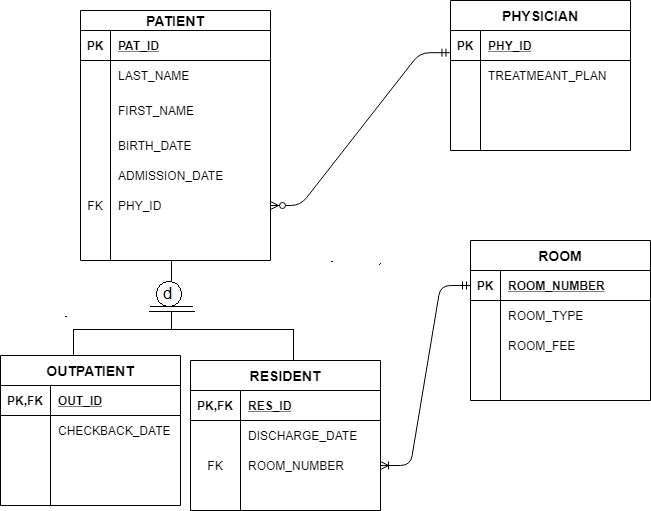
Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy. Well, we use the aufbau principle, and for sulfur, Z=Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15).
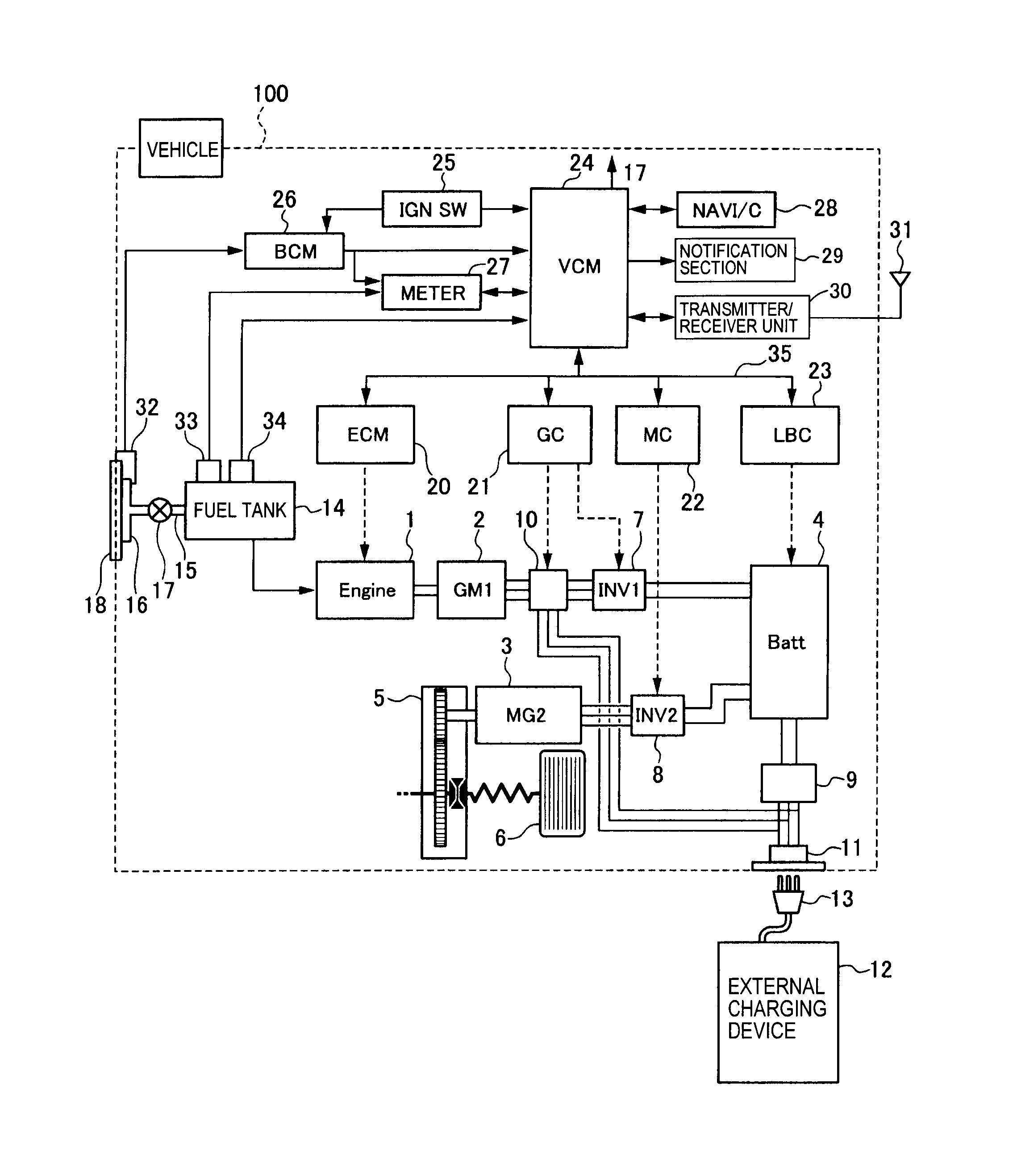
Mar 23, · Show the orbital-filling diagram for (sulfur). Stack the subshells in orderof energy, with the lowest-energy sub shell at the bottom and thehighest-energy subshell at the top.

Show the orbital-filling diagram for (bromine).Status: Resolved. 1.
Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis.
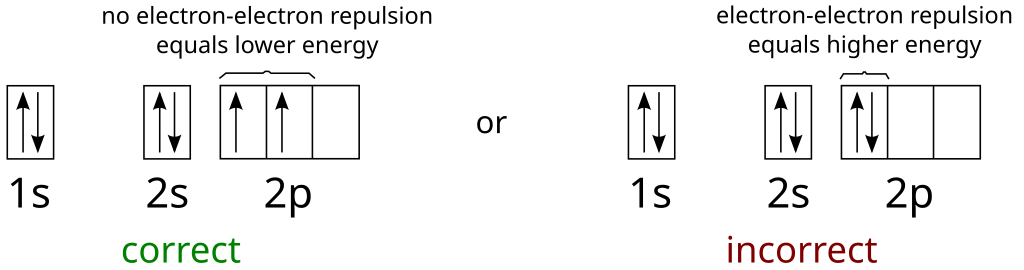
The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus.
What does this tell us about electrons in p orbitals? The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each.
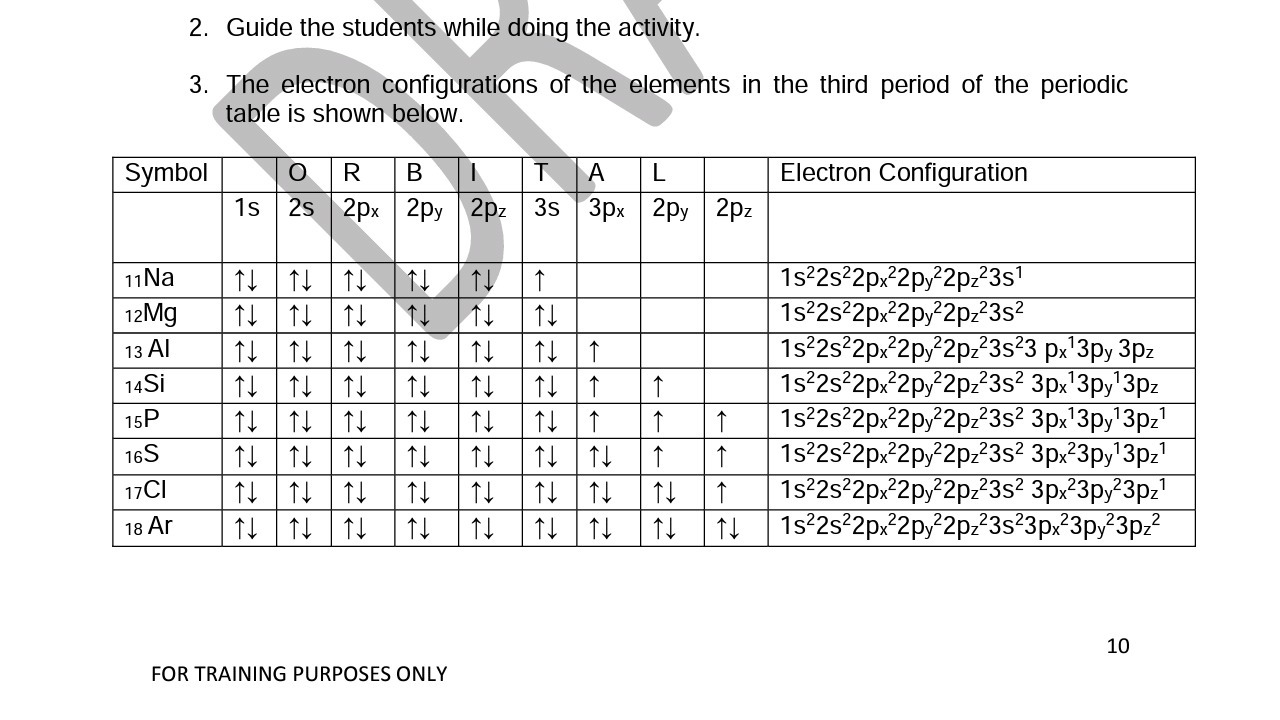
The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur’s orbitals. Sulfur’s electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
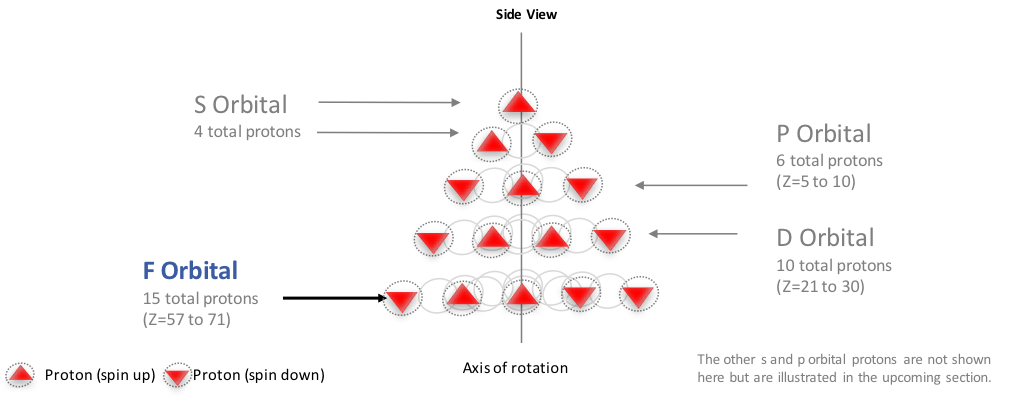
Sulphur/Sulfur (S) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.How many unpaired electrons does an atom of sulfur have in its ground state? | SocraticElectron Configuration for Sulfur (S)