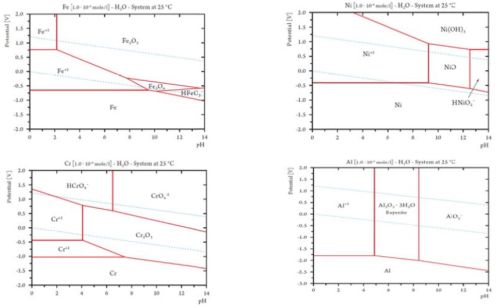
Chemical and electrochemical equilibria are summarized elegantly in a Pourbaix diagram, which is a potentialpH diagram. The diagram is a map of the.
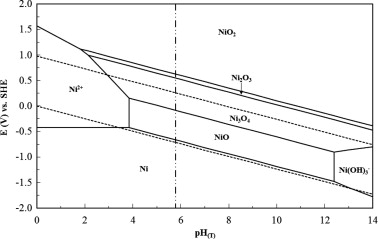
to obtain accurate ΔfG values, which lead to Ni Pourbaix diagrams that are more Ni Pourbaix diagrams have been simulated many times over. The Pourbaix diagrams (potential-pH diagrams) for nickel at 25– °C have been revised.
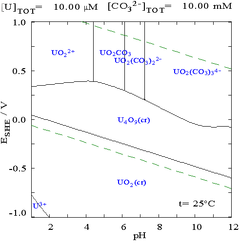
Extrapolation of thermodynamic data to elevated temperatures have. The variants of Pourbaix.
diagram for nickel, available in. literature [1–3], don’t correspond.
CHEM3006 – 30 – Pourbaix diagrams: what are they?
to each other and don’t reflect the. nonstoichiometry.
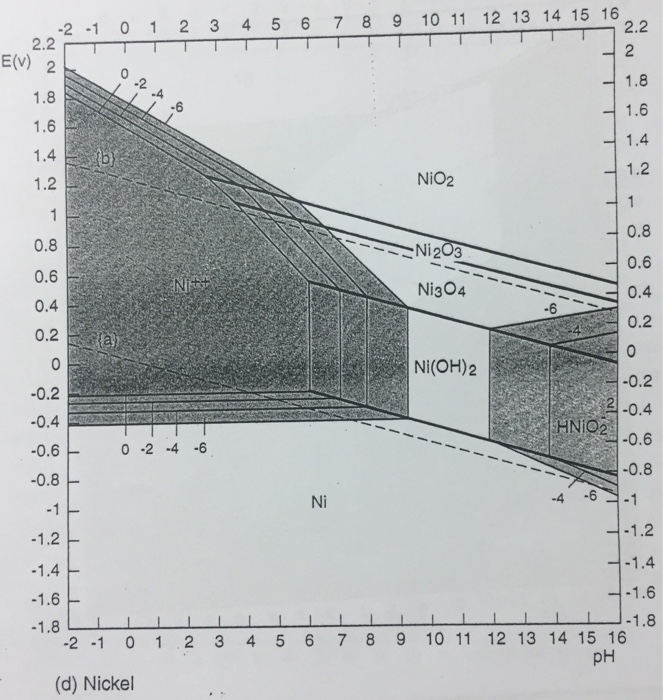
Answer to Using the Pourbaix diagram for nickel, Figure d, give the anodic and cathodic reactions on Ni in water for the follo.Abstract Pourbaix diagrams (potential-pH diagrams) for the ternary system of Fe-Cr-Ni at 25°C to °C were calculated. Extrapolation of thermochemical data to elevated temperatures was performed with the revised model of Helgeson-Kirkham-Flowers, which also allows uncharged aqueous complexes to .
well as surface characteristics of materials. Eh-pH diagrams are thus essential to understanding solute and radionuclide transport in groundwater. The most well-known studies on comprehensive Eh-pH diagrams are those of Pourbaix () and Brookins ().
The former discussed corrosion, passivation and immunity of materials, while the. The Pourbaix diagram can be thought of as analogous to a phase diagram of an alloy, which plots the lines of equilibrium between different phases as temperature and composition are varied.
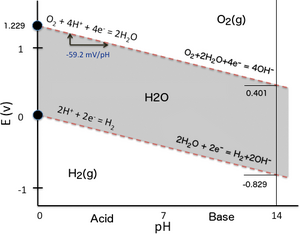
To plot a Pourbaix diagram the relevant Nernst equations are used. Pourbaix Diagrams for Iron.
pourbaix 2016
7 Galvanic Corrosion. Atlas(Eh Ph Diagram) Denny a.
Jones Principles and Prevention of Corrosion Ni Partial Pourbaix Diagram for Ni2+ + 2e- = Ni 3 Documents Similar To 3 2 Pourbaix Diagram. 06 Pourbaix Diagrams.
There was a problem providing the content you requested
Uploaded by. Anonymous T02GVGzB.
Pourbaix Diagram. Uploaded by. agnyreza. The Pourbaix diagrams (potential-pH diagrams) for nickel at 25– °C have been revised. Extrapolation of thermodynamic data to elevated temperatures have been performed with the revised model of Helgeson-Kirkham-Flowers, which also allows uncharged aqueous complexes, such as Ni(OH) 2 (aq), to be handled.Pourbaix diagrams for the nickel-water system extended to hig..|INISPourbaix diagram – Wikipedia