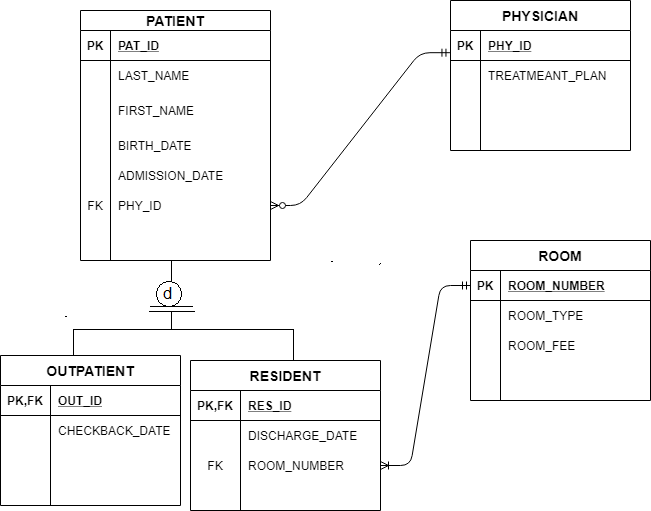
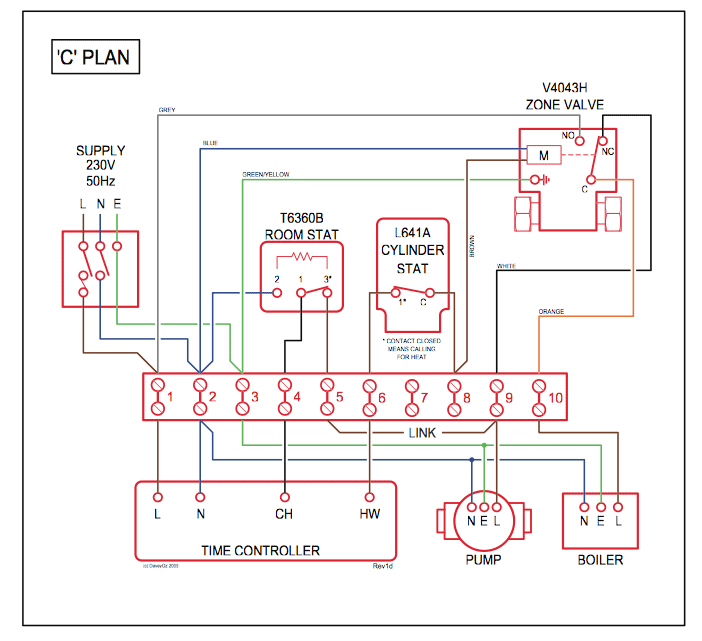
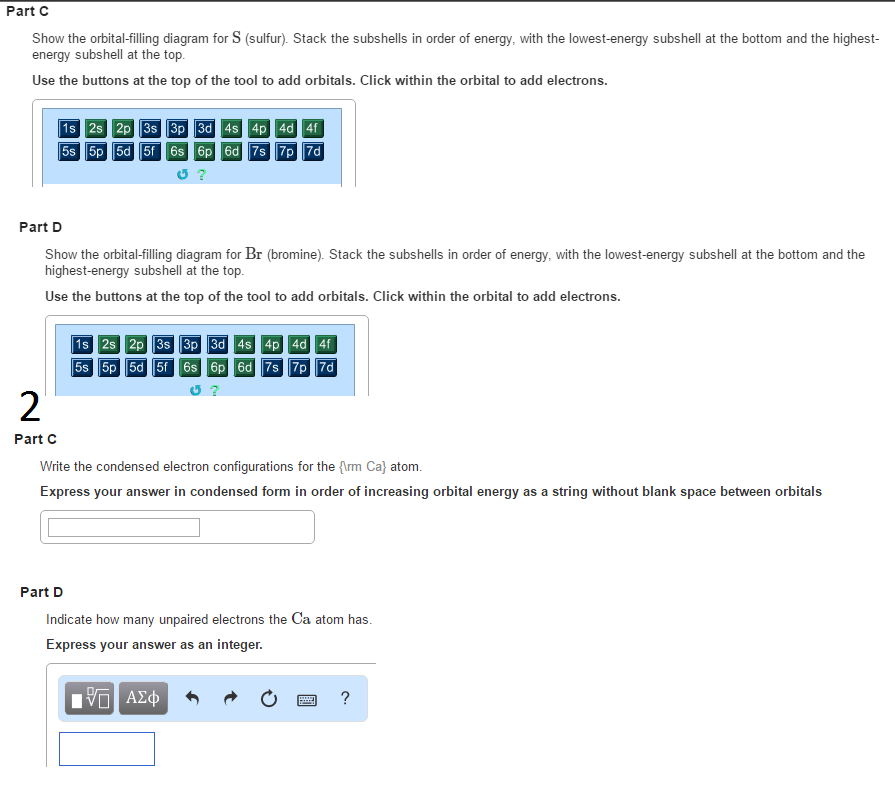
Answer to Show the orbital-filling diagram for (bromine).
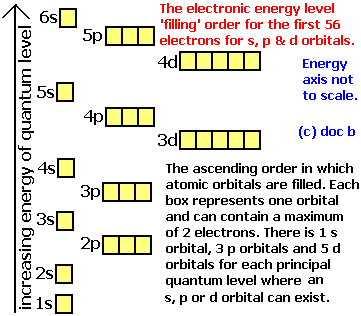
Stack the subshells in order of energy, with the lowest-energy sub shell. sodium. Is°’ 35 iron.
Is7 p6 3s2 3 4s 3. 3) bromine. |s* 26°p33° 3pºus30 ° 4) barium 3p.
AS. 3d.

Ig. 2.
Which element has the following orbital diagram? 3.

Using arrows, show how the following orbitals will fill with electrons. Br. 5.
a. What are valence electrons?
Oukur en ergy level electrons b. Explain how an.
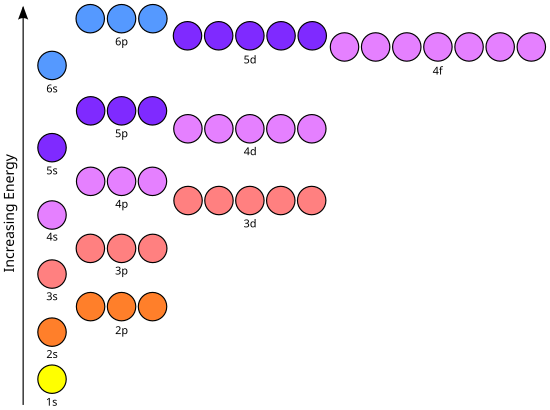
Orbital-Filling Diagram for Bromine. Bromine has 35 electrons, so it will have 35 arrows placed in its orbital-filling diagram as in figure The order bottom to top .
Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full version of this is. Bromine (Br) has an atomic mass of Find out about its Orbital Diagram.
1s. ↿⇂.
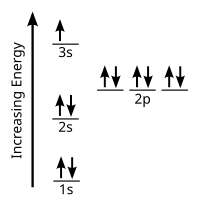
2s. ↿⇂.
2p. ↿⇂ Planetary Bohr Model of Bromine (Br). Lewis Dot Diagram .Chapter 2: Atoms and the Periodic Table This content can also be downloaded as an printable PDF, adobe reader is required for full functionality.
This text is published under creative commons licensing, for referencing and adaptation, please click here. Atomic Theory with Historical Perspectives Introduction to Elements and the Periodic Table Dmitri [ ].How many orbitals are there in the third s | Clutch PrepCH Chapter 2 – Atoms and Periodic Table – Chemistry