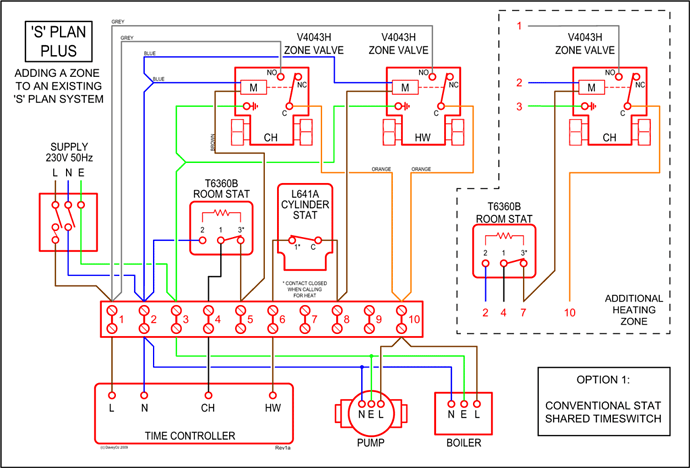
Here are the steps I follow when drawing a Lewis structure. How to analyze different ways to draw the dot structure for sulfur dioxide. A step-by-step explanation of how to draw the SO2 Lewis Structure.
help at http ://schematron.org For the SO2 Lewis struc Easy: Examples and Tricks for Drawing Lewis Dot Diagrams of Molecules. Decide which is the central atom in the structure. That will normally be the least electronegative atom (S).
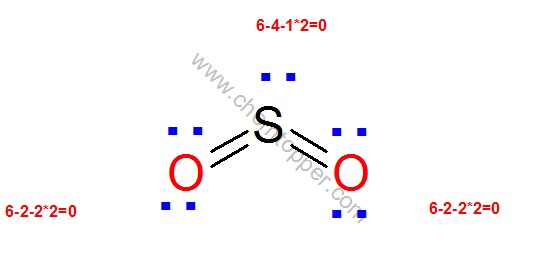
* Draw a skeleton structure in which. How to draw the Lewis Structure of SO2 – with explanation Check me out: http:// schematron.orgDrawing the Lewis Structure for SO 2.
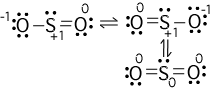
Viewing Notes: The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S).; You might think you’ve got the correct Lewis structure for SO 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons.
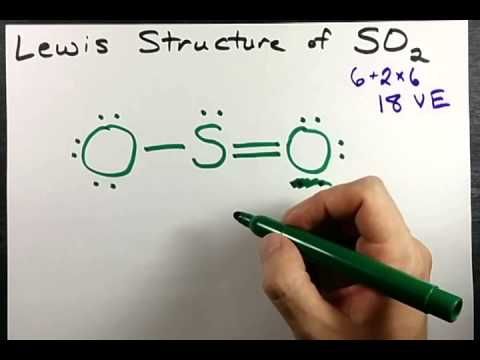
Here are the steps I follow when drawing a Lewis structure. > 1.

Decide which is the central atom in the structure. That will normally be the least electronegative atom (“S”). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: “O-S-O”.
3. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
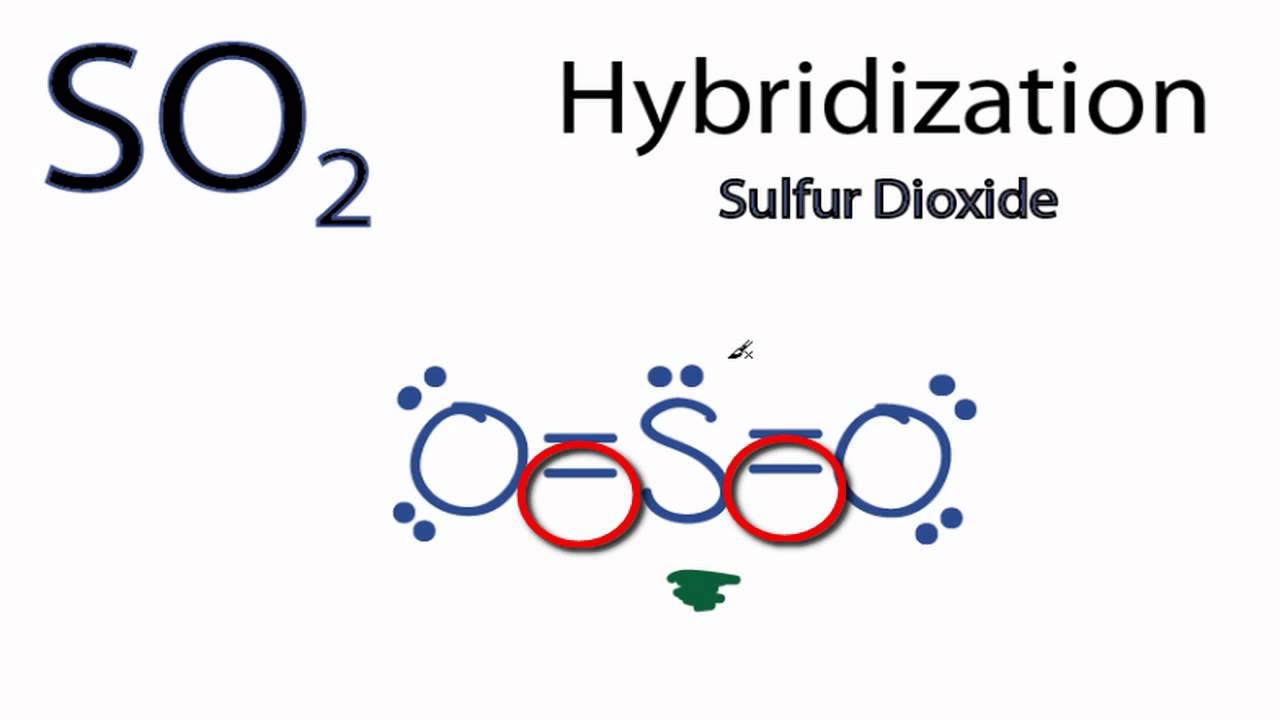
Sulfur dioxide may be shipped domestically via air (cargo only), rail (cargo only), road, and water, in containers bearing the label, “Nonflammable gas.” Sulfur dioxide should be stored in tightly closed containers, in cool, well-ventilated areas, and away from sources of physical damage. For a dot diagram, maybe you don’t have to include molecular geometry. Since this one is simple and also shows something helpful, I’ll put it in anyway.
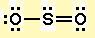
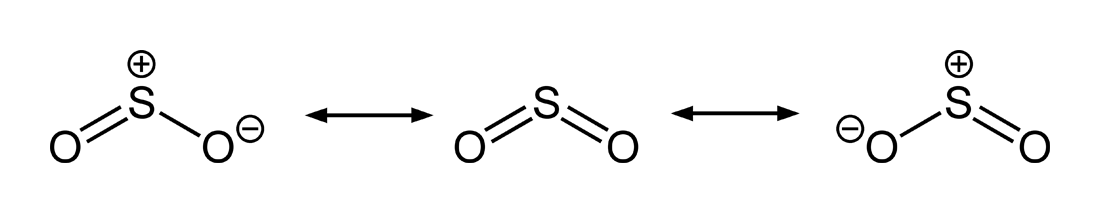
SO2 is a resonance structure. SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure by Janice Powell · Published May 9, · Updated November 6, The Sulfur Dioxide which is also known as Sulphur Dioxide is the entity of a bond between Sulfur and Oxygen atoms.Lewis Dot of Sulfur Dioxide SO2Sulfur dioxide | SO2 – PubChem