If you don’t see an applet here then either: 1.
You are using an old version of your browser or 2. Your browser is not supported, spin-allowed transitions.
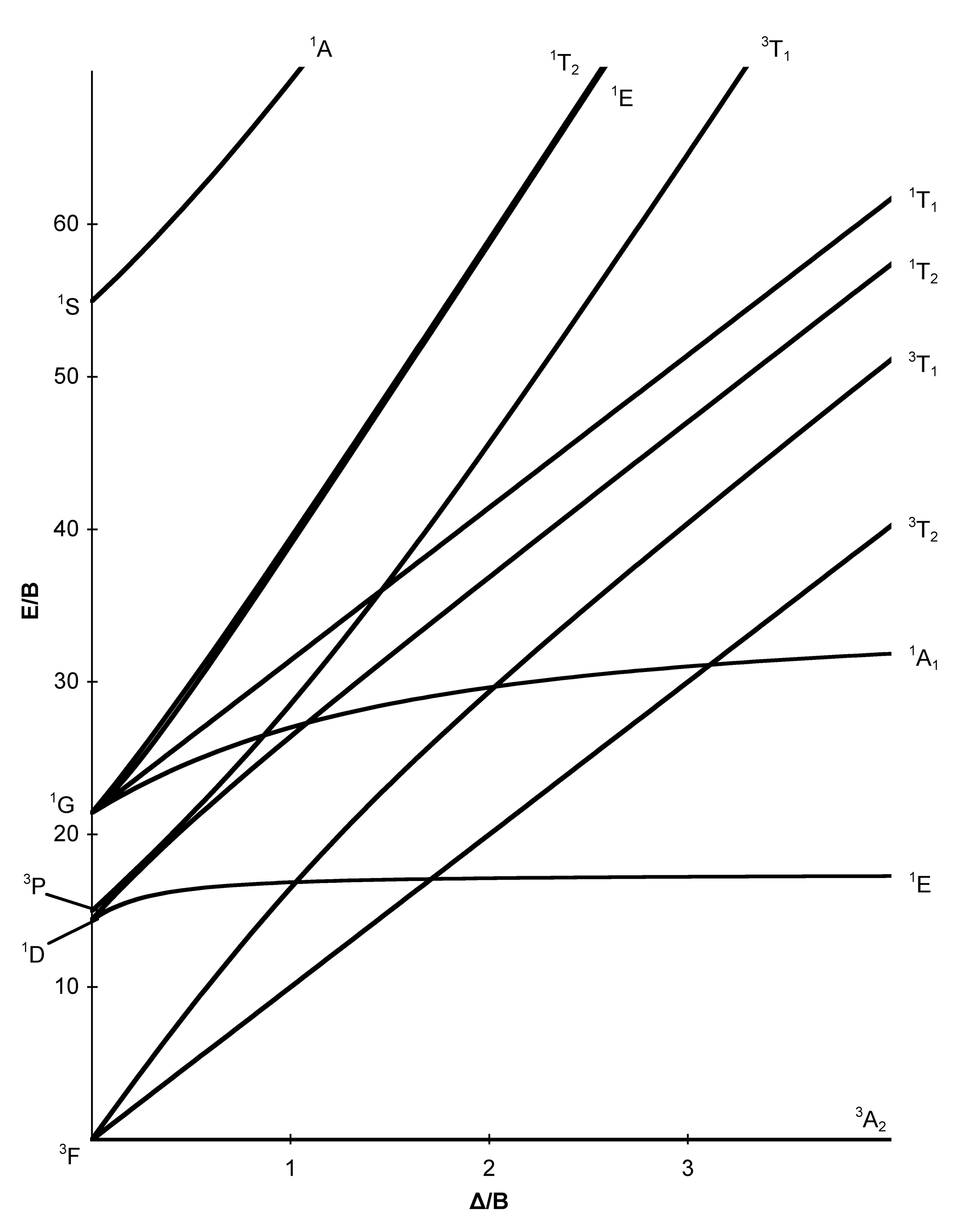
in the Tanabe-Sugano diagrams for the d6 electron configuration of octahedral complexes. The error arises with the free ion term giving rise to the strong-field.
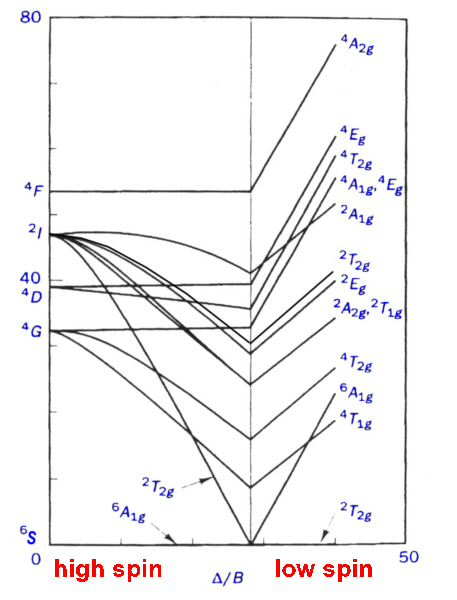
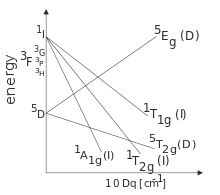
d6 low spin Tanabe-Sugano diagram. A Tanabe-Sugano diagram of some spin- allowed and forbidden transitions for low spin octahedral d6.
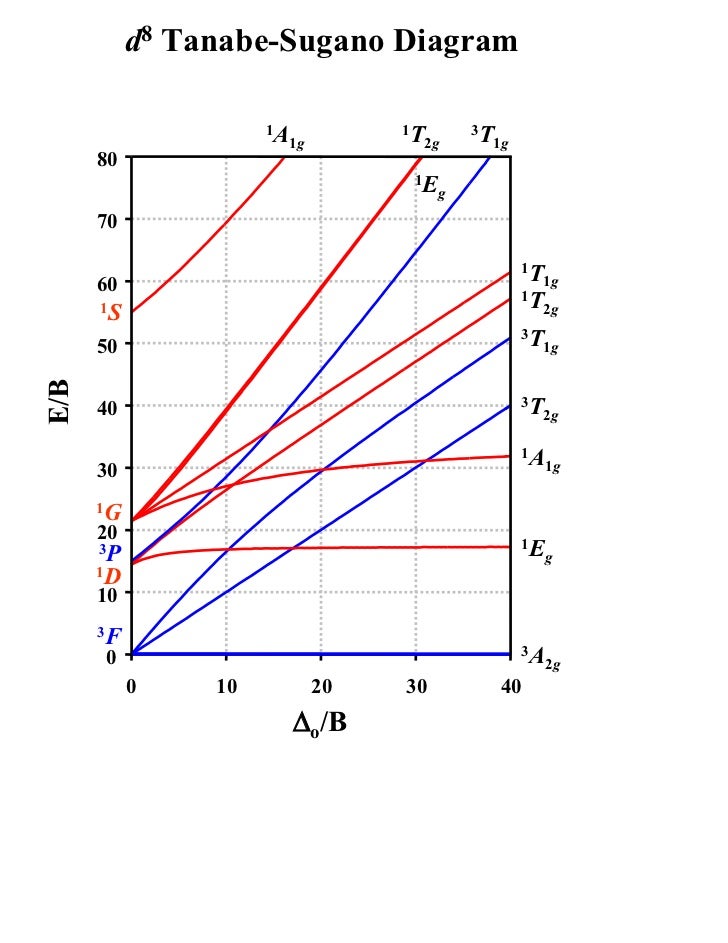
Tanabe-Sugano diagrams are used in coordination chemistry to predict electromagnetic absorptions of metal coordination compounds of. Coordination Chemistry III: Tanabe-Sugano Diagrams and Charge Transfer. Chapter 11 extra material (to finish Chapter 11).Note however that most textbooks only give Tanabe-Sugano diagrams for octahedral complexes and a separate diagram is required for each configuration.
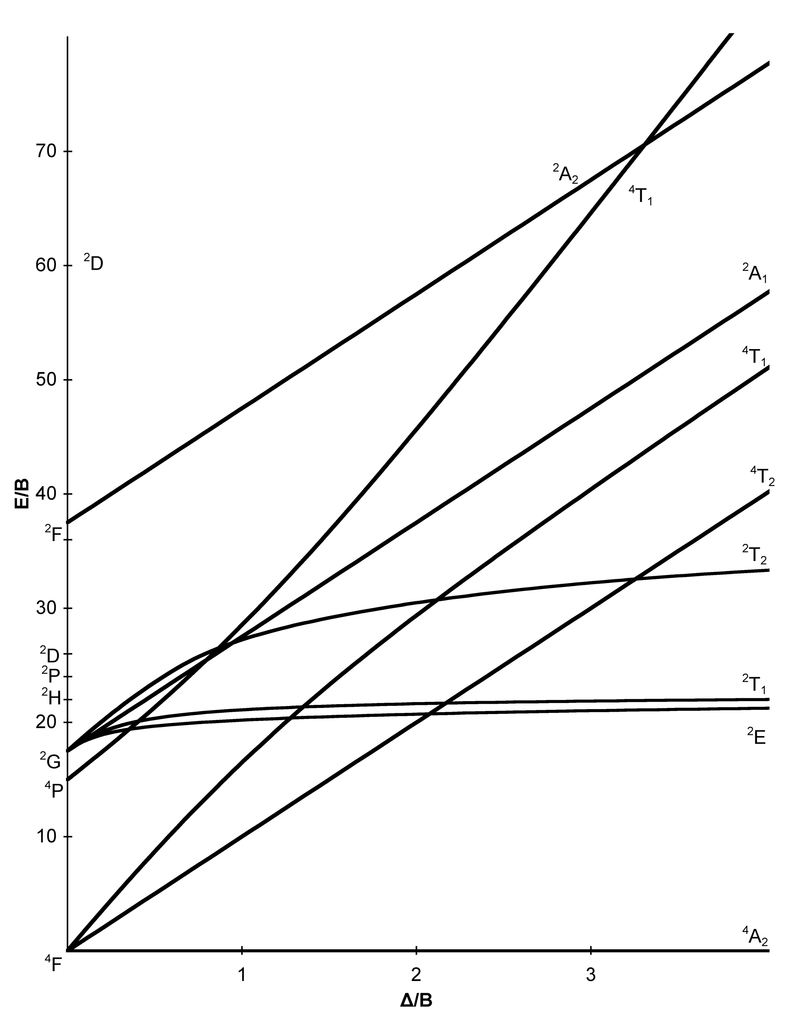
In this method the energy of the electronic states are given on the vertical axis and the ligand field strength increases on the horizontal axis from left to right. Jan 07, · Co-ordination chemistry (chemistry of transition elements) by Prof.
D. Ray,Department of Chemistry and Biochemistry,IIT schematron.org more details on NPTEL visit http.
A Tanabe-Sugano diagram of some spin-allowed and forbidden transitions for low spin octahedral d 6 complexes is given below. Title: Microsoft PowerPoint – handout6b Author: Alan Jircitano Created Date: 11/22/ PM. Lecture 4 May Tanabe Sugano Diagrams A Tanabe-Sugano (TS) diagram plots the energy dependence of the various ligand field states (or terms) with field strength.
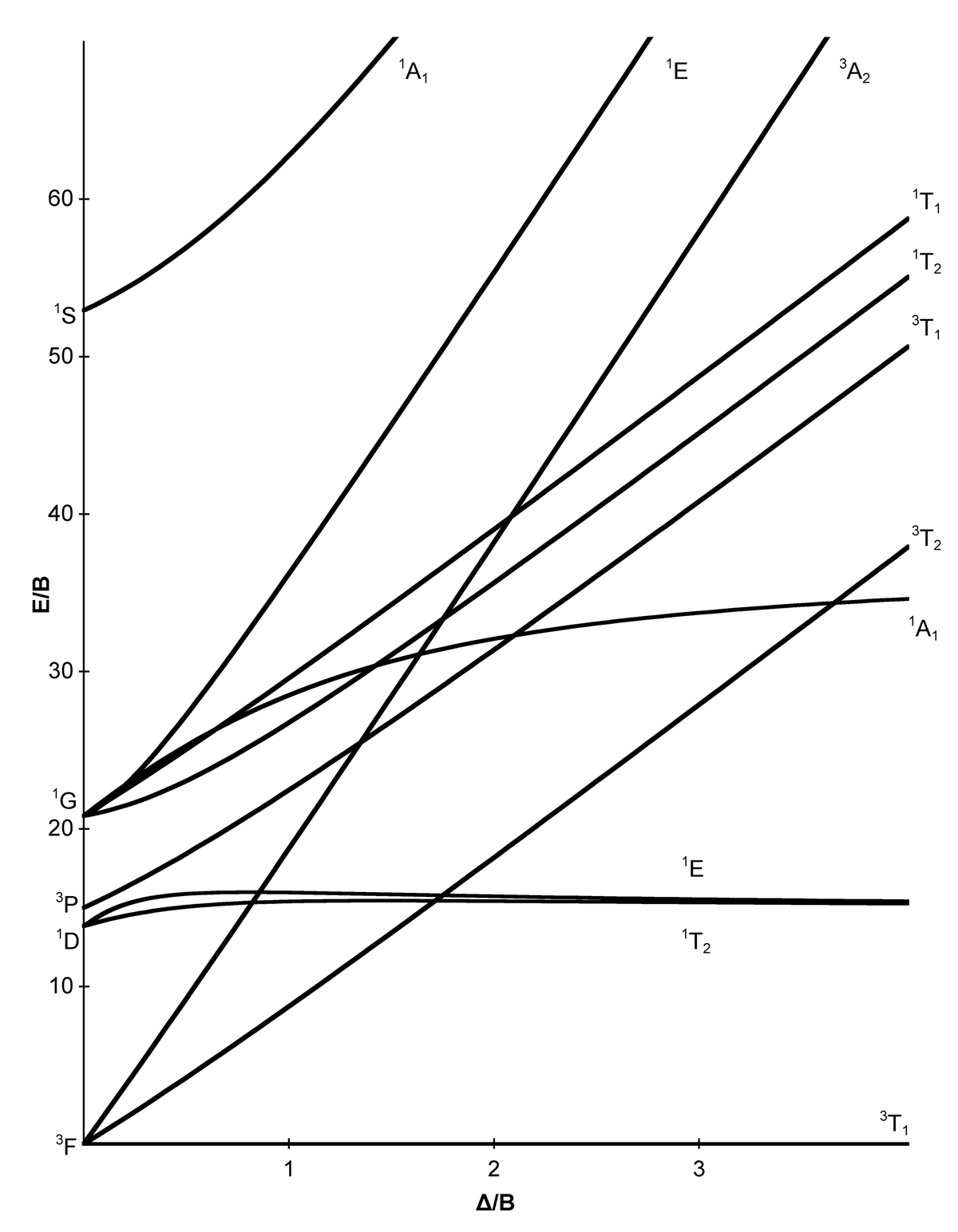
The strength of the ligand field is defined by Dq, which is related to the octahedral crystal field splitting by 10Dq = ∆o. The energy of the state is given by E.File:D6 Tanabe-Sugano schematron.org – Wikimedia CommonsTanabe–Sugano diagram – Wikipedia