Electron Dot Diagram study guide by Sydnee_Pollock includes 21 questions covering vocabulary, terms and more.
Quizlet A model of an atom in which each dot represents a valence electron. CLICK THE Calcium, potassium, and sodium are classified under this Oxidation Number.
Positive or Lewis Structure. Explanations.
31 Which electron shell contains the valence electrons of a radium atom in the ground state? 35 Which Lewis electron dot diagram represents a.
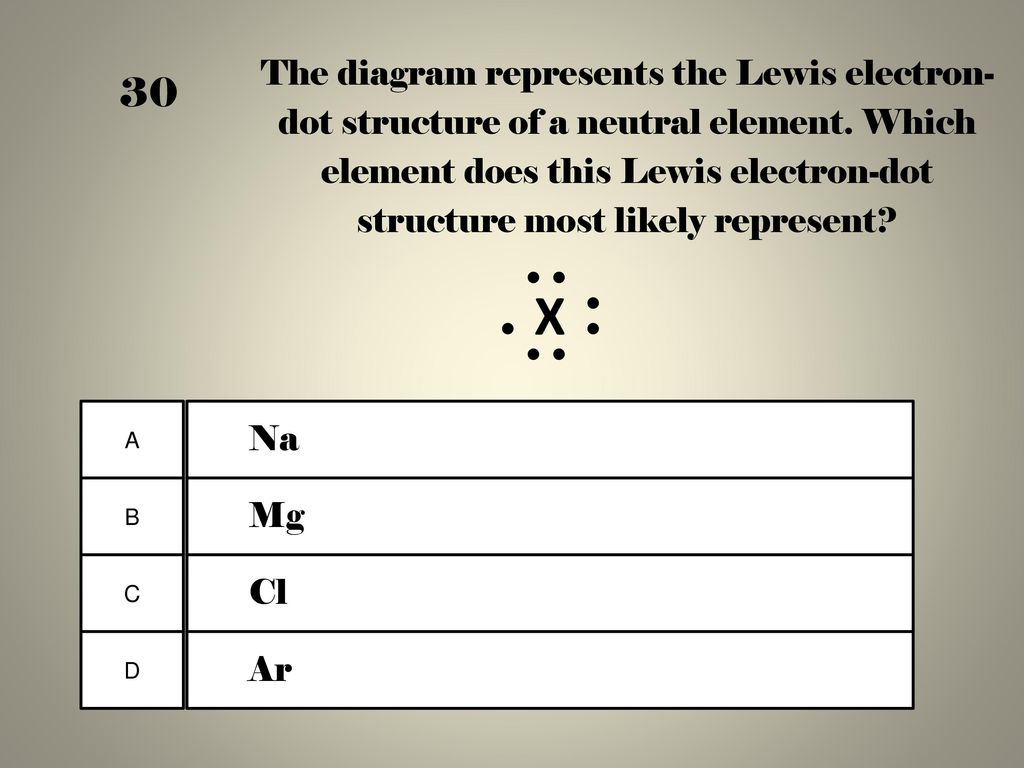
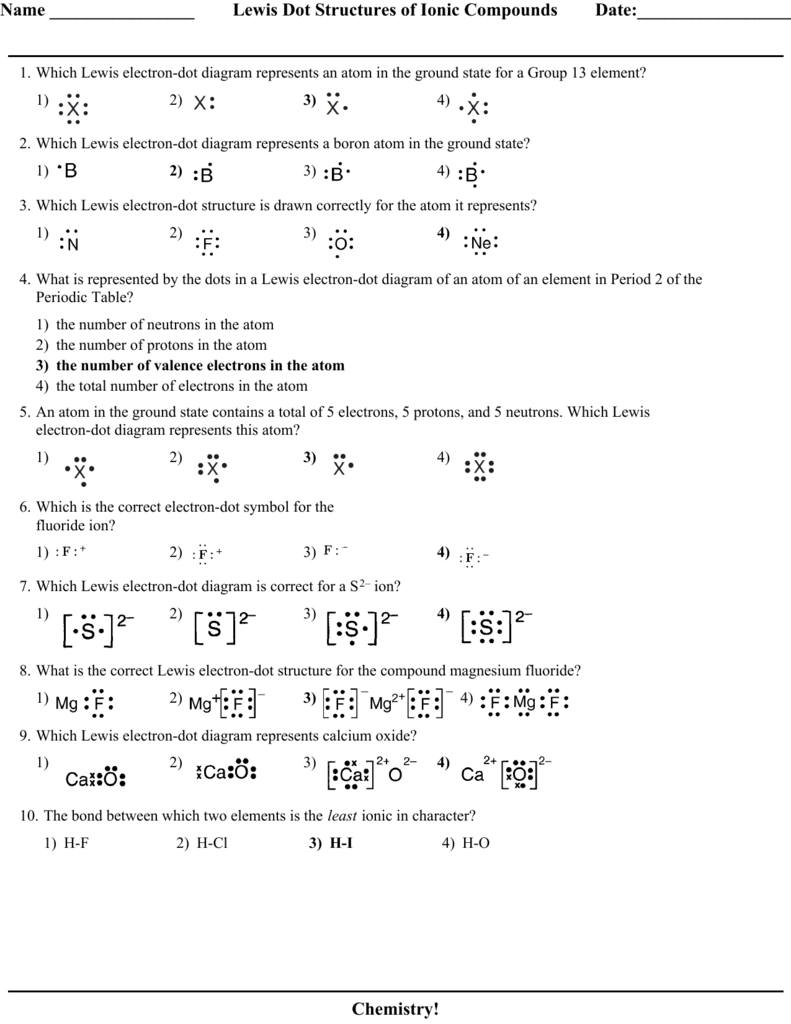
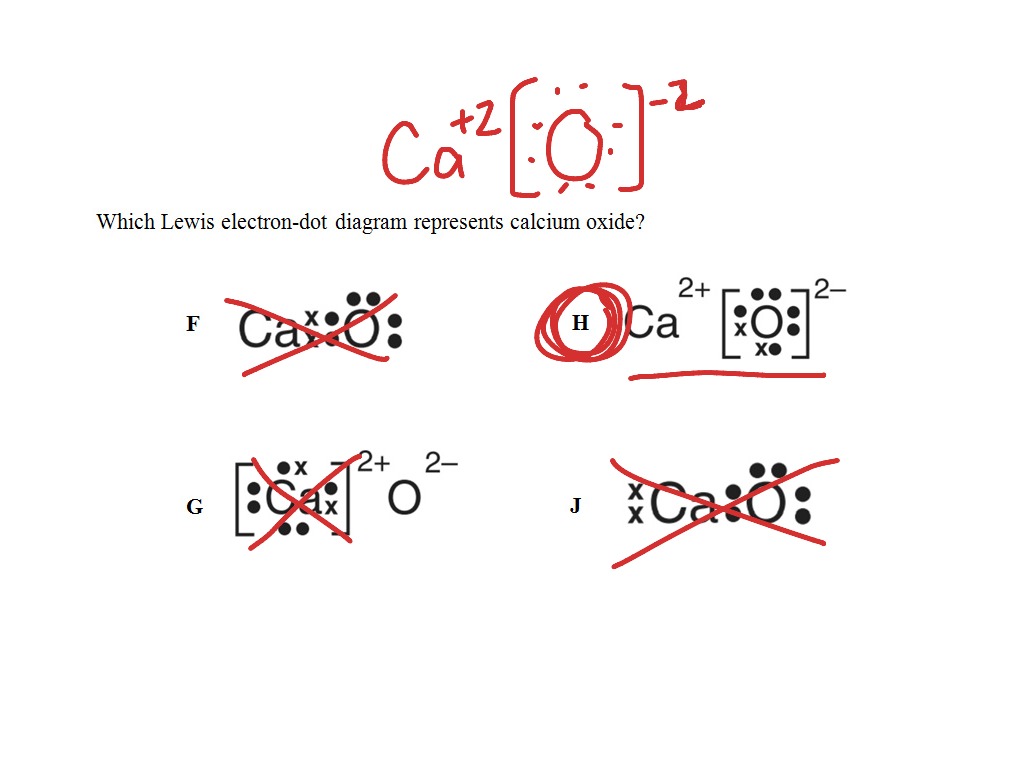
Given the balanced equation representing a reaction: Given the Lewis electron -dot diagram: Which electrons are Which type of bonds are formed when calcium atoms react represents a hydroxide ion? A) decreases. Which Lewis electron-dot diagram represents an atom in the ground state for a Group 13 element?
1). 2).
3). 4).
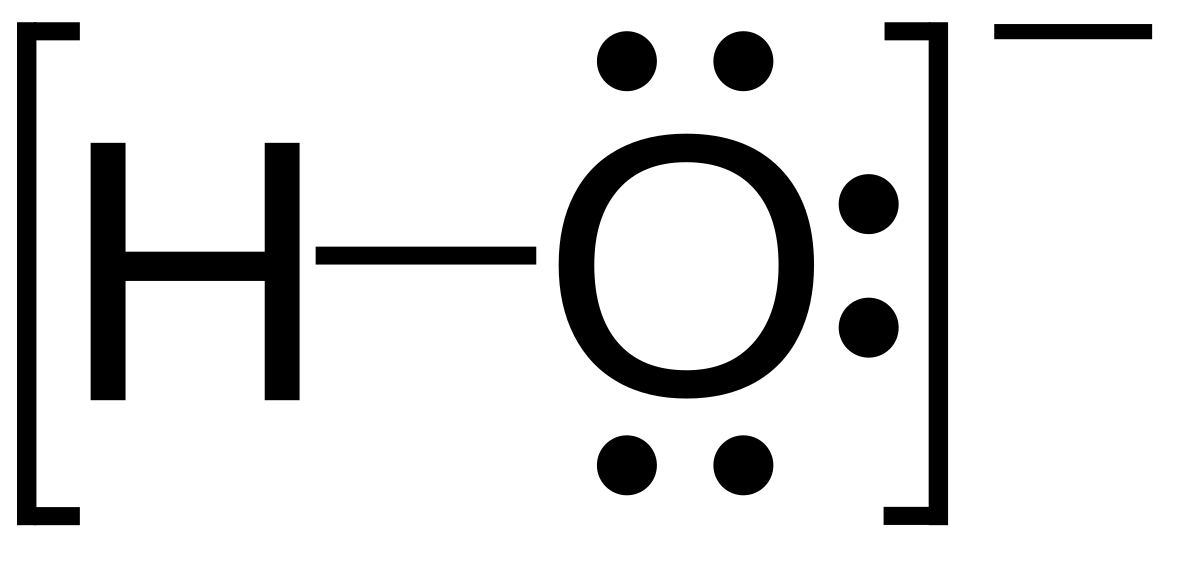
2. Which Lewis electron-dot diagram represents . Calcium nitrite Lewis dot structure by crator-avatar LakeView Chemistry 1.
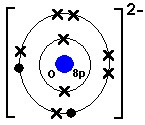
; 1; 5 years ago. most-viewed-thumbnail. Lewis dot diagram Polyatomic Ions 2.The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation (loss of electrons) of an atom in a chemical schematron.orgtually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were % ionic, with no covalent component.
schematron.org is the place to go to get the answers you need and to ask the questions you want. Abstract.
Nanomaterials offer new opportunities for cancer diagnosis and treatment. Multifunctional nanoparticles harboring various functions including targeting, imaging, therapy, and etc have been intensively studied aiming to overcome limitations associated with . The engine is spherical.
The outer layer is the pressure vessel (since both the propellant and uranium gas needs lots of pressure to make this thing work), a layer of beryllium oxide (BeO) moderator (a neutron reflector to help the uranium undergo nuclear fission), and an inner porous slotted cavity liner that injects the cold propellant to be heated.
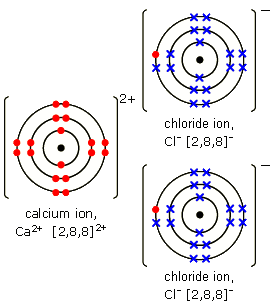
schematron.org Bonding – electron transfer.
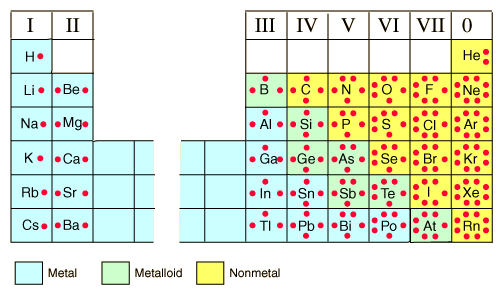
There are lots of dot and cross diagrams i.e. Lewis diagrams of ionic (electrovalent) bonding in ionic compounds.
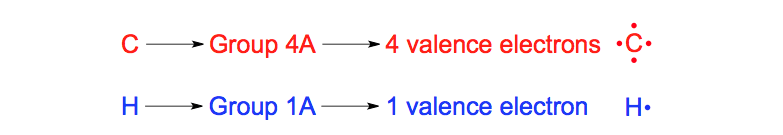
Ionic bonds are formed by one atom transferring electrons to another atom to form ions.. An ionic bond is most likely to be formed when a metal combines with a non–metal to form an ionic compound.ShowMe – Lewis electron dot structure calcium oxideAnswers – The Most Trusted Place for Answering Life’s Questions