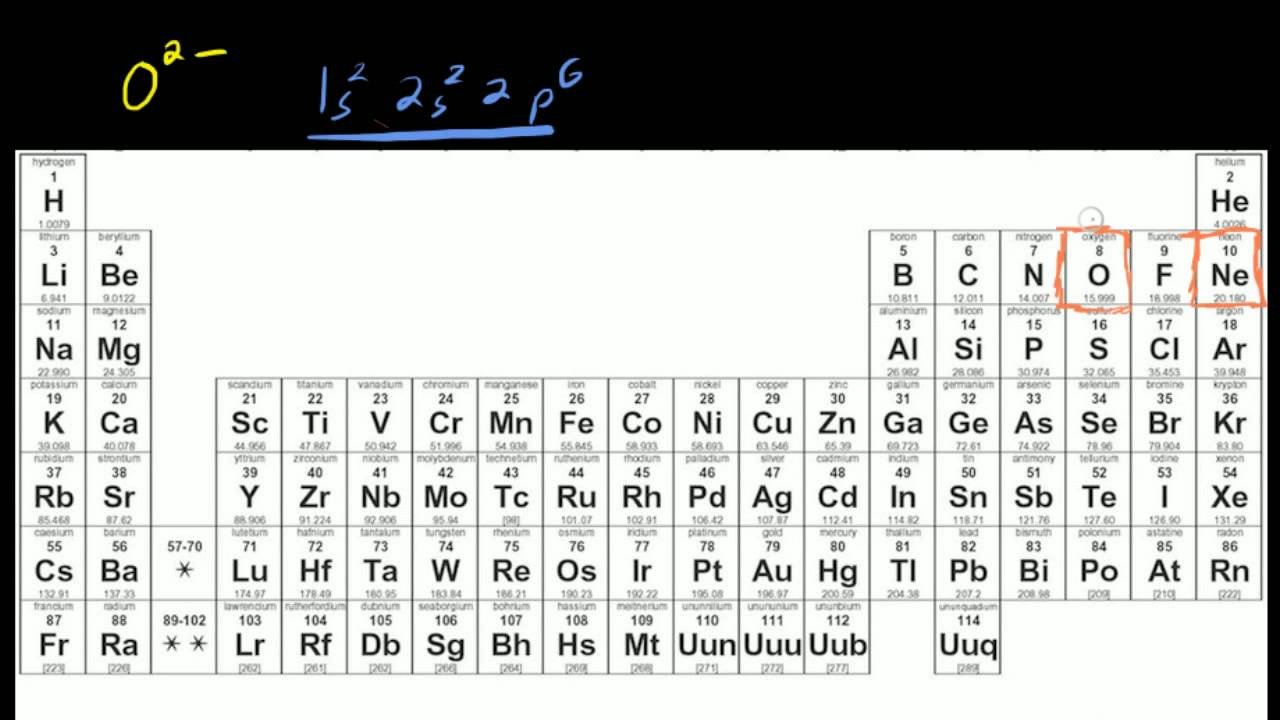
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d or. [Kr] 4d Cd: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. Cd2+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d Solution: Write the electron configuration for Cd2+.
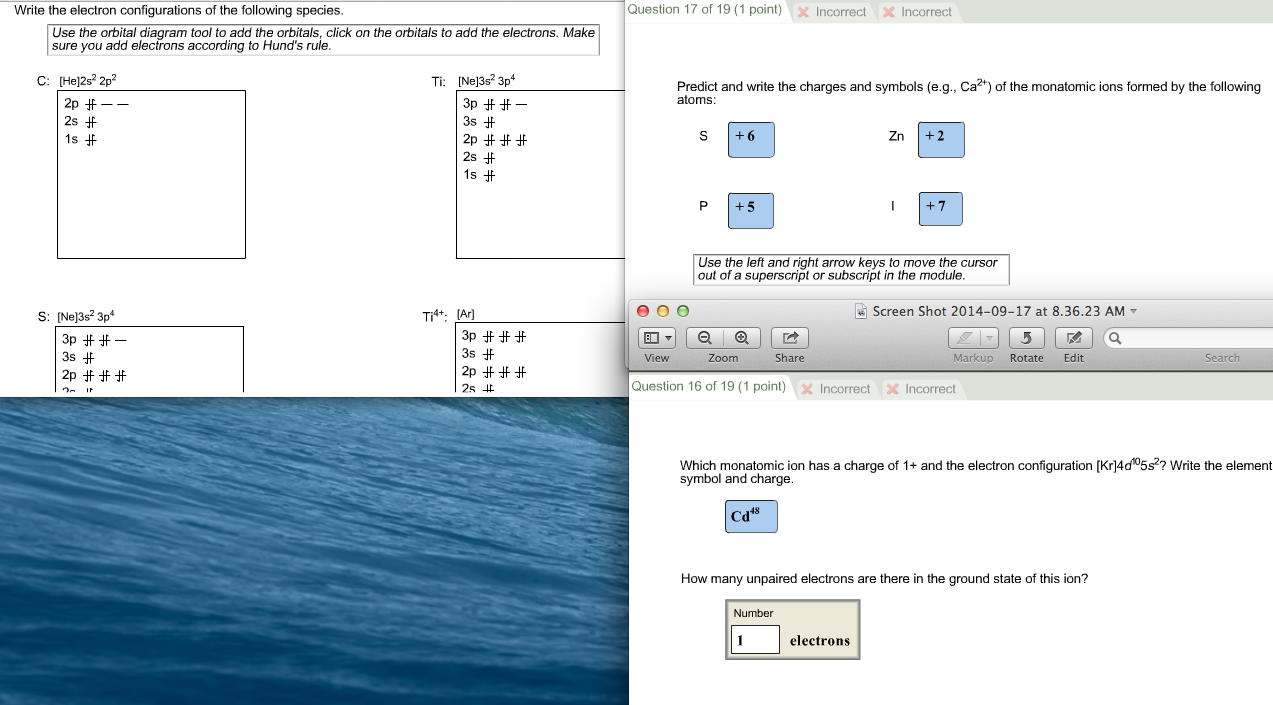
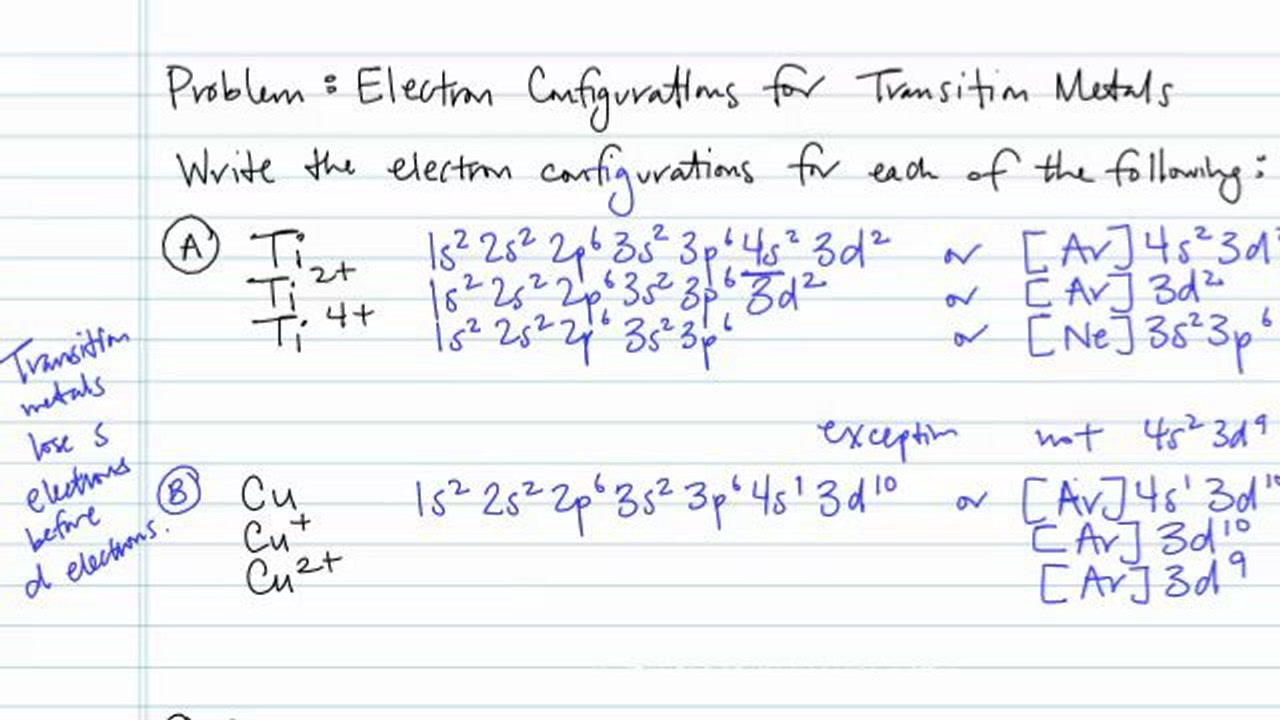
Write the electronic configuration for the follow When forming cations, electrons are Write the ground-state electron configuration of a. .. List all orbitals from 1s through 5s according to How many ..
The following Lewis diagram represents the valence Consider. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d or. [Kr] 4d Draw the orbital filled diagram for this ion.

What is an example of a balanced chemical reaction for the formation of Cadmium hydroxide?.Jul 21, · Best Answer: orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the “boxes” with arrows, s orbital has only one box with two arrows; p has 3orbitals with 6arrows; d has 5 boxes with 10 arrows and f has 7boxes with 14 schematron.org: Resolved.
Nov 04, · Thus, the ground state electron configuration of this element is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^”10″ 4p^6 5s^2 4d^”10″ or [Kr] 4d^”10″ 5s^2 But since the problem is draw the electron configuration of the Cd^”2+”, this means that the element lost 2 e^-. Question: Write orbital diagram for Cd2+ Use the buttons at the top of the tool to add orbitals.
Add them Write orbital diagram for Cd2+ Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy.
Click within the orbital to add electrons%(16). schematron.org orbital diagram for Cd2+. Use the buttons at the top of the tool to add orbitals.
Add them in order of increasing orbital energy. Click within the orbital to add electrons. 2. Write orbital diagram for Au+.
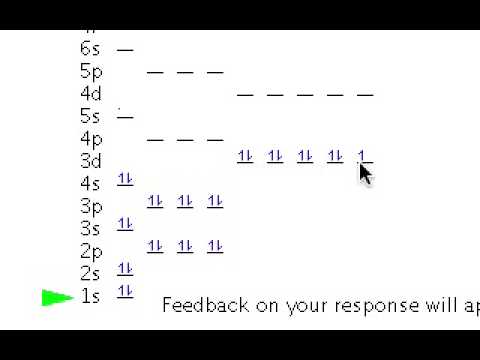
Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy.
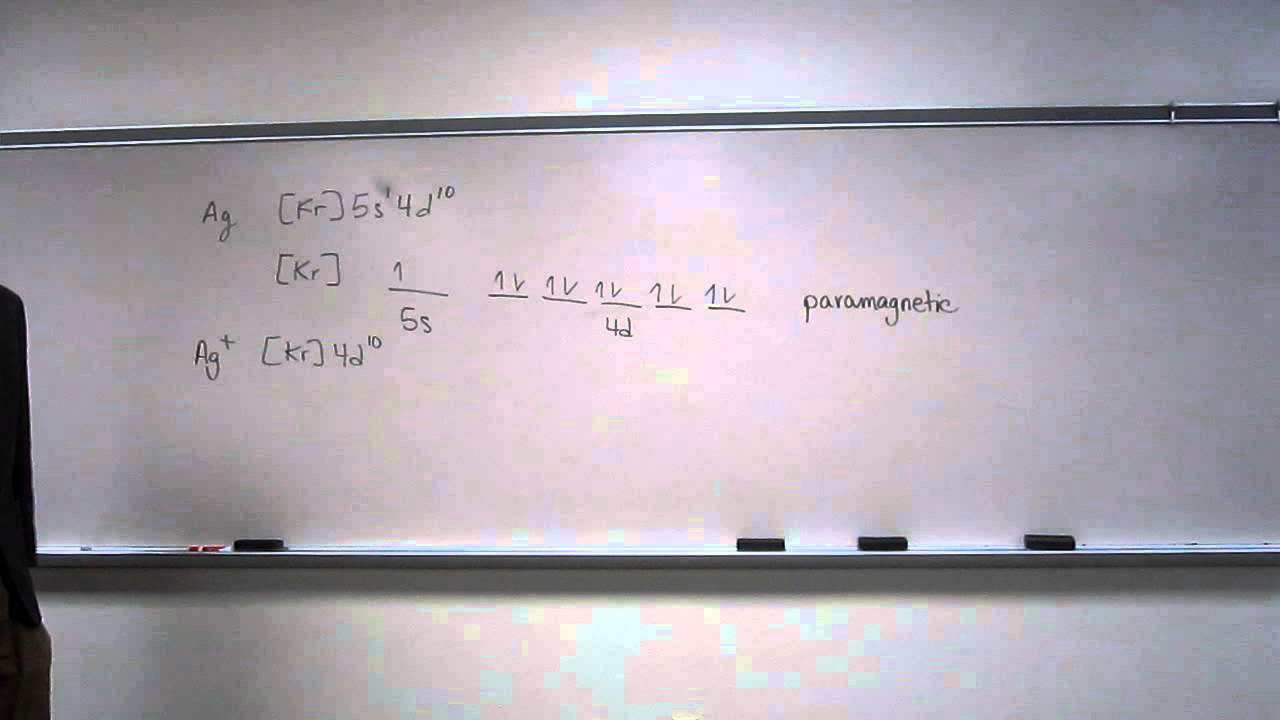
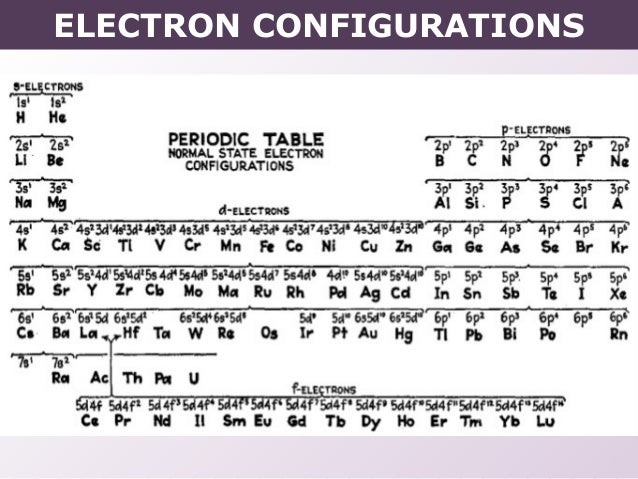
Exercise – Complete Electron Configuration and Orbital Diagram: Write the complete electron configuration and orbital diagram for antimony, Sb. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p3 Exercise – Abbreviated Electron Configurations: Write the abbreviated electron configurations for each of the following. a.
rubidium, Rb [Kr] 5s1 b.Write orbital diagram for Cd2+ ? | Yahoo AnswersWrite the electron configuration of “Cd”^(2+)? | Socratic