For efficient experiments, it’s essential to familiarize yourself with the 96-cavity layout used in laboratory settings. This format is ideal for high-throughput screening, allowing you to conduct multiple tests simultaneously with minimal sample volume. Each section of the grid is meticulously designed to maximize space while maintaining ease of use in automated systems.
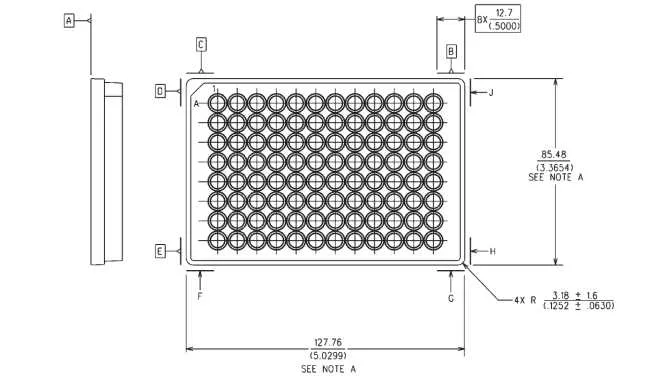
Each of the 96 compartments is arranged in an 8×12 pattern, enabling quick identification and access. Rows are typically labeled with letters from A to H, while columns are numbered from 1 to 12, facilitating precise placement and retrieval of samples. This systematic design is crucial for maintaining consistency across experiments and ensuring accurate data collection.
When preparing for experiments, consider the volume capacity of each compartment, usually between 100 µL to 500 µL. This ensures efficient reagent use and effective sample manipulation. Additionally, be mindful of the material and coating of the grid, as these factors influence sample interaction and experiment outcomes.
Key Tip: Always ensure proper sealing or covering of each section to avoid contamination or evaporation during incubation. This practice is particularly vital when conducting time-sensitive or temperature-dependent tests.
96-Pocket Array Layout
For efficient sample organization in multi-well containers, arrange the compartments into an 8×12 grid. This layout maximizes space while ensuring easy access and accurate identification. Label rows with letters (A to H) and columns with numbers (1 to 12) to facilitate tracking and avoid mix-ups. Ensure each pocket is adequately spaced for pipetting and optical measurements, considering the standard 6.4 mm distance between centers of adjacent compartments.
When using this setup for assays, consider the specific volume capacity of each unit–usually around 200 µL. This will allow for optimal performance during experiments involving dilution, PCR, or ELISA. For consistency, maintain a consistent orientation of the container during handling and imaging to ensure reproducibility.
Tip: Utilize a color-coded system to denote different sample types or reagents, enhancing both organization and speed of data interpretation.
Note: Always avoid overfilling any compartment to reduce the risk of cross-contamination between adjacent samples, especially when dealing with high-throughput testing protocols.
Understanding the Layout of a 96-Well Plate for Laboratory Experiments
Efficient organization is crucial for maximizing experimental throughput and accuracy. Here’s a breakdown of how to effectively utilize a 96-position format in laboratory setups:
- Rows and Columns: The structure consists of 8 rows (A-H) and 12 columns (1-12). Labeling each position with a combination of row letter and column number, such as A1, B3, and so on, ensures clarity in identifying specific spots.
- Capacity: Each space holds 100–200 µL of liquid. This volume is ideal for various assays, including ELISA, PCR, and cell culture testing, providing flexibility for different protocols.
- Consistent Spacing: A consistent gap between each position minimizes cross-contamination risks, ensuring that samples remain isolated from adjacent spots.
- Top-Down Orientation: Always ensure the correct orientation when positioning the setup in your equipment, especially when automating the process. This eliminates the possibility of misalignment during sample analysis.
- Reproducibility: When filling multiple spaces, ensure even distribution. This is vital for reproducibility across tests, especially in assays that depend on precise reagent quantities.
Consider the layout when planning experimental replicates or when dealing with a multi-step process. Design the configuration to minimize movement between tasks and optimize sample preparation efficiency.
How to Use a 96-Well Setup for High-Throughput Screening
Start by preparing all samples and reagents in advance. Ensure each compartment contains the correct volume, usually between 50-200 µL, depending on the assay requirements. Label each row and column for easy tracking of your substances. Always use multichannel pipettes for efficient and consistent liquid handling across multiple locations.
Optimize incubation times based on the specific biological process you are studying. For example, in enzyme assays, incubation times range from 30 minutes to several hours, depending on the reaction kinetics. Use a temperature-controlled shaker to maintain consistent conditions across all compartments.
When performing readouts, choose the appropriate detection system. Optical absorbance, fluorescence, and luminescence are common methods, and your choice should align with the reagents and the assay’s sensitivity. Ensure that the optical path length is consistent for all measurements to minimize variation between tests.
For analysis, employ automated systems to process data from multiple compartments simultaneously. This is crucial to reduce human error and increase throughput. Statistical software can help identify significant hits and assist with hit validation.
Regularly check for contamination or cross-contamination between compartments by employing barrier seals or using dedicated pipetting tips. Minimize evaporation by covering the setup during longer incubation periods, especially when working with volatile compounds.
Optimizing Arrangement for Specific Assays
Prioritize row and column positioning based on assay requirements. For high-throughput screening, use the outer rows and columns to place controls, while keeping experimental samples centralized for uniform conditions. This minimizes edge effects and enhances reproducibility.
For serial dilution assays, align samples along the first few columns, ensuring accurate pipetting without cross-contamination. Maintain consistency by using even-numbered rows to avoid any variability that may arise from adjacent well differences.
For kinetic or time-dependent assays, place samples in adjacent positions across the same row or column. This allows for simultaneous measurement and reduces potential errors caused by variations in incubation time across different locations.
When using a multi-step assay, organize the plate so that reagents and samples are placed according to the order of addition. This helps maintain accuracy and reduces potential errors during the pipetting process.
Temperature-sensitive experiments should allocate samples in central locations to ensure even thermal distribution. Outer sections may be slightly affected by environmental changes, so keep sensitive reactions away from edges.