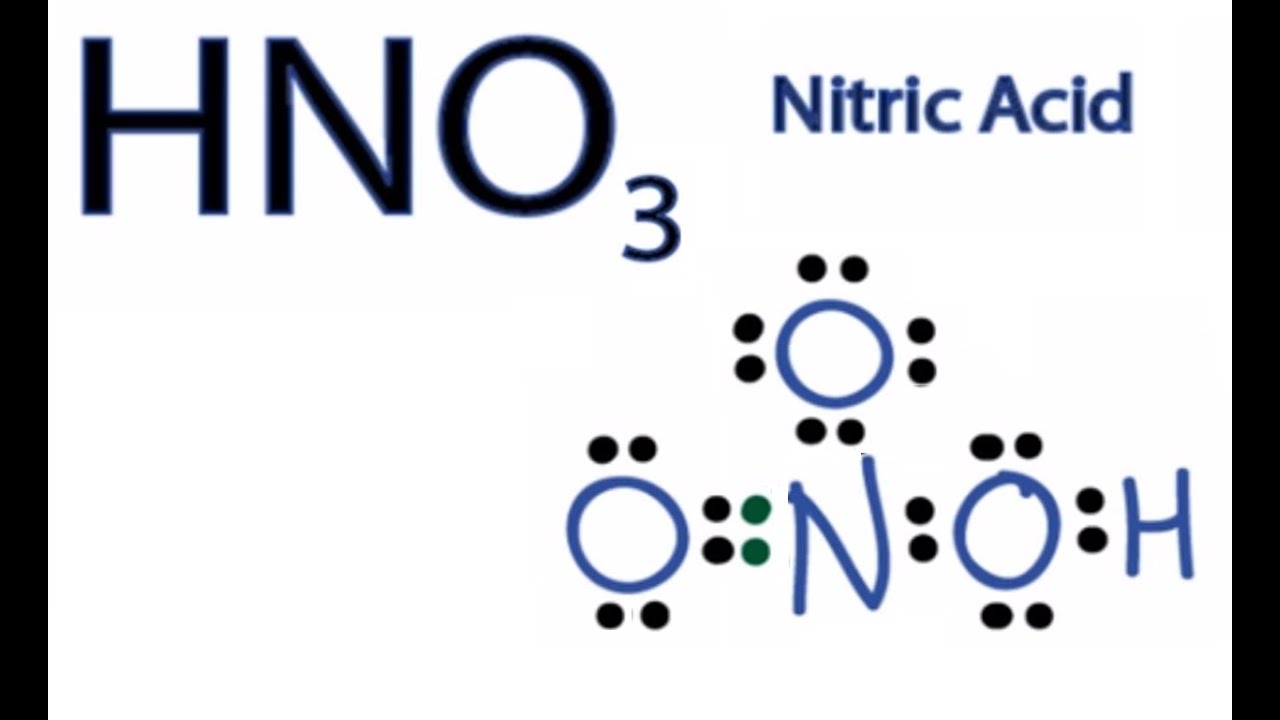
How to analyze different ways to draw the dot structure for sulfur dioxide. Here are the steps I follow when drawing a Lewis structure.
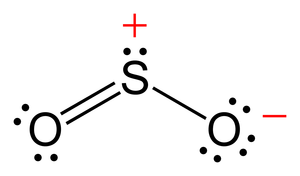
How to draw the Lewis Structure of SO2 – with explanation Check me out: http:// schematron.org The “best” Lewis structure is one in which has the fewest formal charges. We can generate a structure with zero formal charges if we move a.
A step-by-step explanation of how to draw the SO2 Lewis Structure. Get more chemistry help at schematron.org For.SO2 Lewis structure.
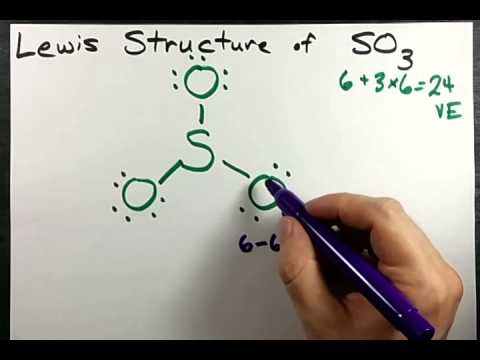
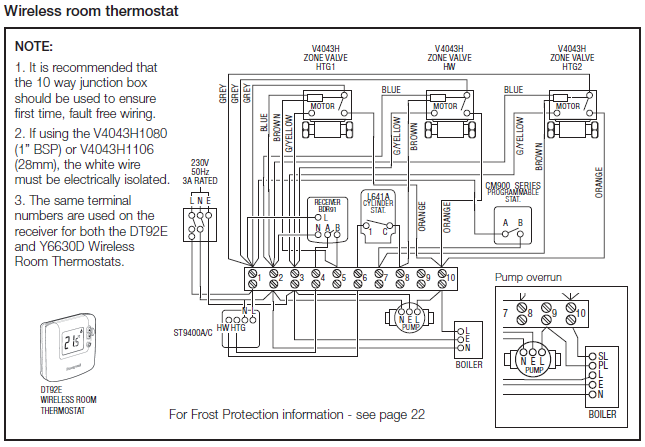
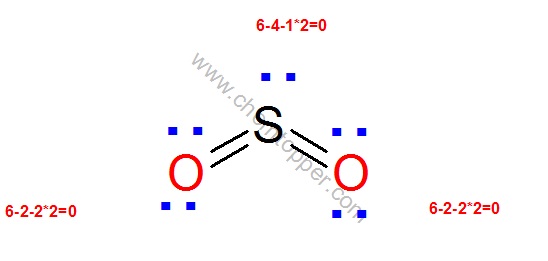
To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur. To design the best Lewis structure, you also need to calculate the formal charge of every atom too.
You know that both the Sulphur and Oxygen has six valence electrons each. 70 More Lewis Dot Structures.
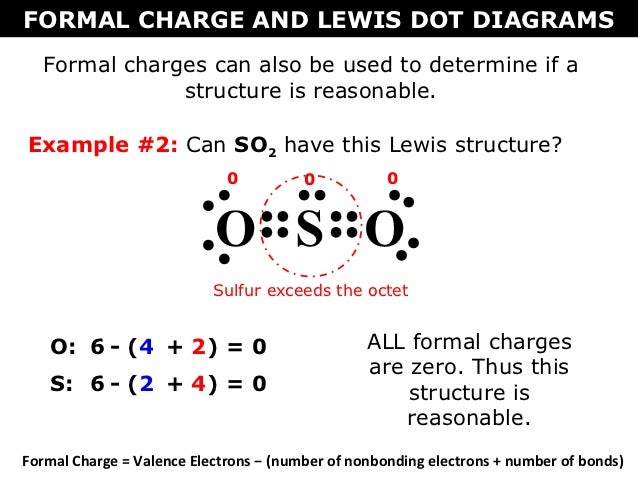
S does not follow the octet rule. It can hold more than 8 electrons.
Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. Lewis Dot of Sulfur Dioxide.
SO 2. Back: 70 More Lewis Dot Structures.
S does not follow the octet rule. It can hold more than 8 electrons.
Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. NOT IN THIS MOLECULE.
The Lewis dot structure of SO2, or sulfur dioxide, has a central atom of sulfur that violates the octet rule. The central atom of sulfur has one lone pair and is double bonded to two oxygen atoms.
Sulfur has valence electrons in the 3rd energy level, allowing access . Drawing the Lewis Structure for SO 2.
Viewing Notes: The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S).; You might think you’ve got the correct Lewis structure for SO 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons.Covalent| Lewis structure| SO2 – Simple Procedure for Dot structures – #53 | Chemistry NetLewis Dot of Sulfur Dioxide SO2