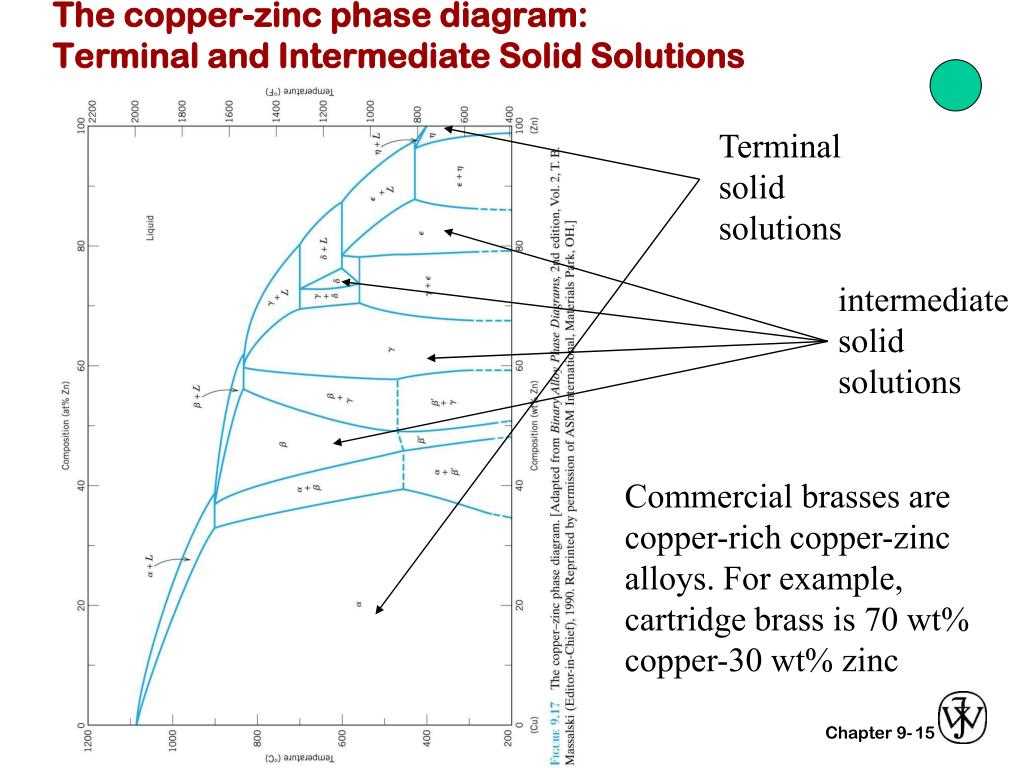
The Copper Tin Phase Diagram describes the relationship between the composition and temperature of an alloy made from copper and tin. It provides valuable information about the different phases that metals can exist in at various temperatures.
At lower temperatures, the Copper Tin Phase Diagram shows that the alloy is in a single solid phase known as the α-phase. This phase is characterized by a homogeneous mixture of copper and tin atoms, resulting in a solid solution with a unique crystal structure.
As the temperature increases, the Copper Tin Phase Diagram shows that the alloy undergoes a phase transformation and enters a two-phase region. This region consists of both the α-phase and another phase known as the β-phase. The β-phase is characterized by a different crystal structure and a different composition than the α-phase.
At even higher temperatures, the Copper Tin Phase Diagram shows that the alloy transitions into a single phase region once again. This third region is known as the γ-phase, which has properties distinct from both the α-phase and the β-phase. Understanding these phase transformations is crucial for controlling the properties of copper-tin alloys and predicting their behavior under different conditions.
Background
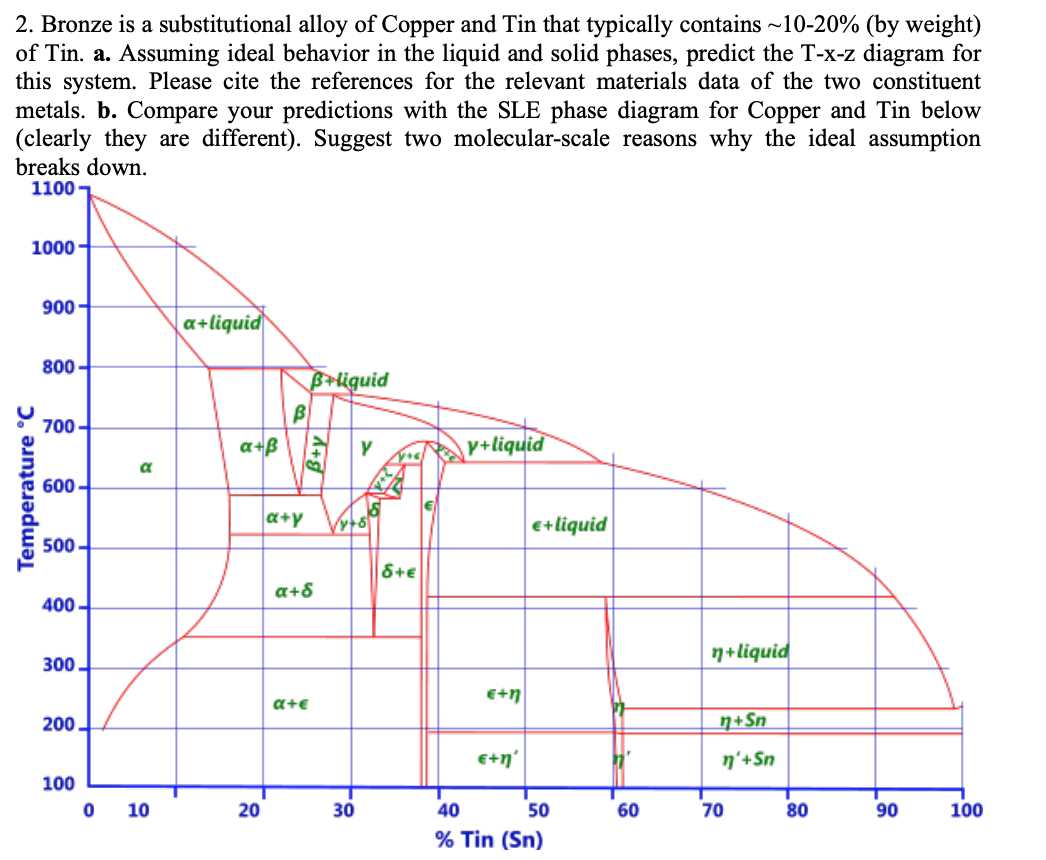
The copper-tin phase diagram is a graphical representation of the relationship between temperature and composition in a binary alloy system of copper and tin. It shows the different phases that can form under different conditions of temperature and composition, providing important insights into the properties and behavior of copper-tin alloys.
Copper and tin are both metallic elements that can form solid solutions with each other. The phase diagram allows us to understand how these solid solutions form and transform into other phases, such as intermetallic compounds or eutectic mixtures, depending on the temperature and composition of the alloy.
One of the key features of the copper-tin phase diagram is the presence of a eutectic point, which represents the lowest melting point of the alloy. At this composition, the two elements combine to form a mixture with unique properties, such as improved strength and hardness. Understanding the behavior of the eutectic phase is crucial for the casting and manufacturing processes of copper-tin alloys.
Phases
- α (alpha) phase: This is the solid solution phase of copper and tin at low temperatures, where tin atoms are dispersed in the copper lattice. The composition of the alloy determines the amount of tin atoms present in the α-phase.
- β (beta) phase: This is another solid solution phase of copper and tin, but with higher concentrations of tin. The β-phase can form at higher temperatures, and its properties are influenced by the composition and cooling rate of the alloy.
- Eutectic phase: This is the phase that forms at the eutectic composition. It consists of a mixture of the α and β phases and has a unique microstructure that contributes to the desirable properties of copper-tin alloys.
- Intermetallic compounds: These are compounds that form when the concentration of one element exceeds certain limits. In the copper-tin system, intermetallic compounds such as Cu3Sn and Cu6Sn5 can form under specific conditions.
The copper-tin phase diagram provides valuable information for metallurgists, materials scientists, and engineers working with copper-tin alloys. By understanding the phase transformations that occur in these alloys, researchers can optimize their compositions and processing conditions to achieve the desired mechanical, thermal, and electrical properties. This knowledge is also important for studying the historical and archaeological significance of copper-tin alloys in ancient civilizations.
Phase diagram
Phase diagram is a graphical representation of the different phases that a substance can exist in under varying conditions of temperature and pressure. It shows the relationships between the different phases of a substance, such as solid, liquid, and gas, as well as the boundaries between these phases.
The phase diagram of a substance can provide important information about its physical properties and behavior. It gives insight into the changes that occur in a substance as it is heated or cooled, and how it transitions between different phases. This can be particularly useful for understanding the behavior of materials in various industrial and scientific processes.
The copper tin phase diagram is a specific example of a phase diagram that shows the relationship between copper and tin alloys. It illustrates the different phases that can be formed, such as alpha, beta, and liquid phases, as well as the boundaries between these phases. This phase diagram is important in the field of metallurgy, as it helps to understand the properties and behavior of copper tin alloys, which are commonly used in various applications, including electrical wiring and plumbing.
Applications
The copper-tin phase diagram has important applications in several industries. Here are some of the key areas where the phase diagram is used:
- Metallurgy: The phase diagram is crucial for understanding the behavior of copper-tin alloys during the manufacturing process. It helps determine the appropriate temperature ranges for solidification, annealing, and other heat treatment processes to achieve desired alloy properties.
- Engineering: The phase diagram is used in the design and production of copper-tin alloys for various engineering applications. These alloys are known for their excellent mechanical properties, corrosion resistance, and electrical conductivity, making them suitable for applications such as connectors, bearings, and electrical wiring.
- Manufacturing: The phase diagram plays a vital role in controlling the composition of copper-tin alloys during the manufacturing of products. It helps ensure the desired phase structure and mechanical properties are achieved, leading to high-quality and reliable products.
- Research and development: The phase diagram serves as a valuable tool for researchers and scientists studying the behavior of copper-tin alloys. It provides insights into the various phases and their transformations, enabling the development of new and improved alloys with enhanced properties.
In summary, the copper-tin phase diagram is essential in metallurgical processes, engineering applications, manufacturing, and research and development. It guides the design and production of copper-tin alloys, ensuring the desired properties are achieved. By understanding the phase diagram, professionals in these industries can optimize their processes, leading to more efficient and high-quality products.