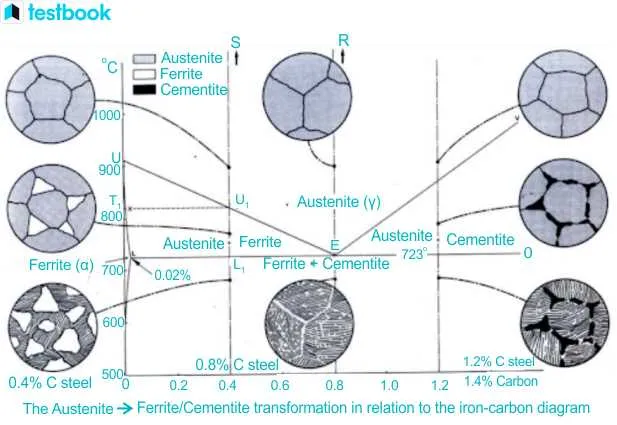
For accurate prediction of material properties in metallurgy, focus on the temperature composition relationships between carbon and its interaction with iron. This is essential for professionals seeking to optimize the creation of alloys with specific mechanical properties. Understand the key phases that emerge as temperature varies, especially in the region where iron alloys transform under different thermal conditions. Pay particular attention to the boundaries where solid phases coexist, as these are crucial for controlling the characteristics of the final product.
Consult the equilibrium curve to identify phase transitions and determine the appropriate cooling rates to avoid undesired structures. The interaction between carbon-rich compounds and metallic solutions significantly alters hardness, brittleness, and strength. The eutectoid point, where transformations complete under normal pressure, is especially important when designing steels or cast irons for heavy-duty applications.
To achieve precision in alloy formulation, consider leveraging these equilibrium compositions. Data from this system aids in crafting tailored materials with the required combination of strength, ductility, and thermal resistance. For professionals in the field, mastering these thermal relations is the foundation for efficient material engineering and reducing trial-and-error in production processes.
Practical Insights on the Fe-Fe3C System
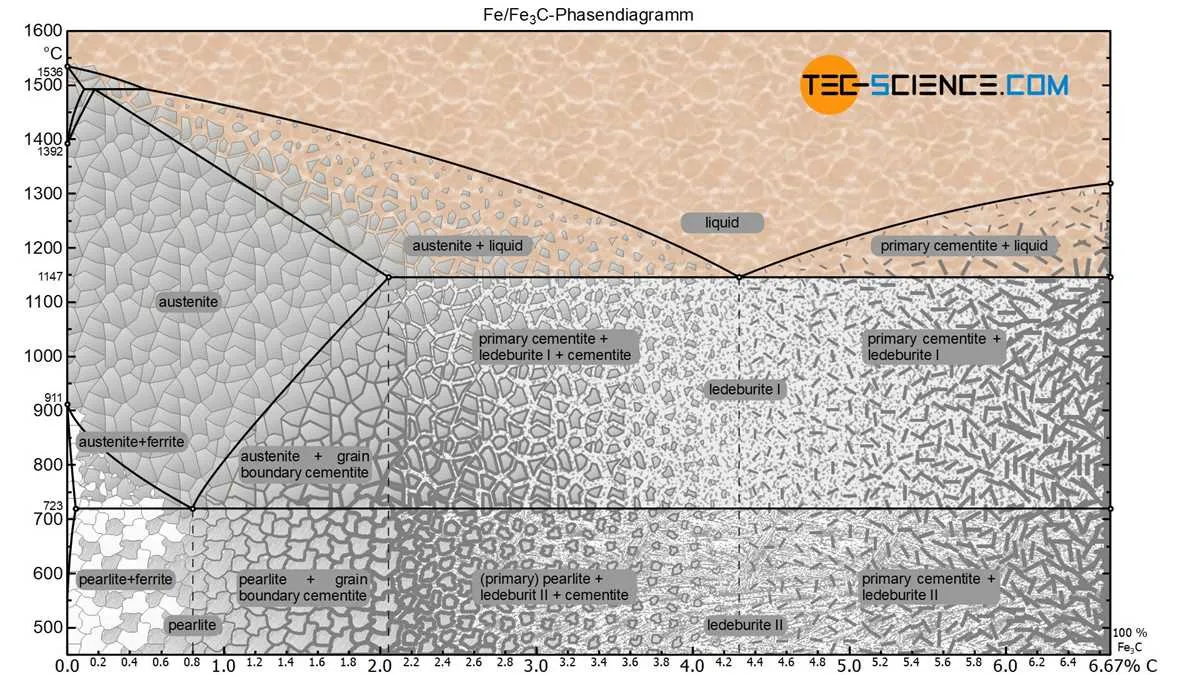
For optimal results in heat treatment and material design, understanding the behavior of alloys in the Fe-Fe3C system is essential. Here are key recommendations for practical applications:
- Critical Temperatures: Focus on the eutectoid composition of 0.8% carbon at 727°C. This point defines the transition between the pearlite and proeutectoid phases, crucial for controlling mechanical properties.
- Leverage the Peritectic Point: At 0.16% carbon and 1493°C, the peritectic reaction marks a significant change in solidification behavior. It’s critical when considering casting or alloying processes where carbon content is low.
- Optimize Quenching: For materials with high carbon content, rapid cooling rates lead to the formation of martensite, which is essential for hardening. However, ensure that cooling rates are managed to avoid cracking in high-carbon steels.
- Transformations in Heat Treatment: When heating the material above the A1 line (727°C), it will dissolve cementite into the solid solution. This is vital for ensuring uniform structure before quenching or further processing.
- Consider Carbon Content for Strength: As the carbon concentration increases, hardness and brittleness rise, while ductility decreases. For engineering components, aim for a balanced carbon level to meet both strength and durability requirements.
Understanding these key aspects enables precise control over the mechanical properties and the behavior of steel during manufacturing processes, ensuring both reliability and performance in the final product.
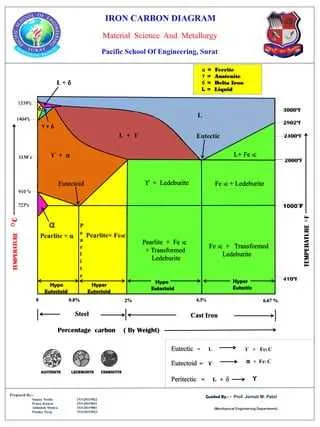
Determining the Temperature and Composition Ranges of the Cementite Phase
To identify the precise temperature and composition ranges for the phase, careful analysis of its boundary lines is essential. The critical temperature for transformation into this structure typically lies between 650°C and 700°C, depending on the exact carbon content present in the alloy. The phase is stable within a specific range of carbon concentrations, generally from 6.7% to 0.8%, with the highest stability at 6.7%. Below this concentration, a different solid structure predominates, while above, the system enters austenitic or other higher-order phases.
Below is a table that outlines key temperature-composition points that help delineate the phase boundaries in an alloy system:
| Carbon Content (%) | Temperature (°C) |
|---|---|
| 6.7 | 1150 |
| 4.3 | 950 |
| 2.0 | 800 |
| 0.8 | 725 |
The boundaries shift depending on the cooling rate, with faster quenching tending to suppress the formation of the phase at lower temperatures. These ranges are used to predict and control the microstructure during heat treatments in practical applications.
Impact of Cementite on Steel Hardness and Strength
The presence of carbides significantly increases the hardness and tensile strength of steel. At higher temperatures, the carbon-rich phase forms a network that restricts the movement of dislocations, thereby improving mechanical properties. When the carbon content in the alloy exceeds a certain threshold, the transformation leads to a more rigid structure, enhancing resistance to deformation under stress. This results in improved wear resistance, making it suitable for high-load applications.
In steel, the volume fraction of carbides directly correlates with hardness. As carbide content increases, the material becomes harder, but at the cost of decreased ductility. For optimal performance in structural applications, balancing carbide concentration is crucial. The presence of these compounds also influences the material’s response to heat treatment; specific temperature and cooling rates can promote carbide precipitation, further enhancing hardness without compromising too much on strength.
Strength increases with the fine dispersion of carbide particles, which act as barriers to grain boundary sliding. However, excessive carbide formation can lead to brittleness, as the microstructure may become overly fragmented. Therefore, controlling the amount and distribution of carbon phases is essential for maintaining a balance between hardness and toughness in engineered steels.
Practical Applications in Heat Treatment Processes
For optimizing the hardness and strength of steel alloys, careful control of temperature and cooling rates is crucial. During processes like tempering or quenching, achieving the desired microstructure requires precise manipulation of the solidification and transformation stages. The eutectoid reaction, which results in the formation of a fine mixture of two distinct solid phases, plays a critical role in determining the mechanical properties of the material. By understanding the transition between different solid forms at varying temperatures, engineers can tailor the final properties of the alloy for specific applications.
In the case of hardening processes, rapid cooling from high temperatures can promote the formation of a high-carbon solid solution, significantly increasing strength but reducing toughness. To restore balance, tempering at moderate temperatures allows for the transformation into a more stable configuration, improving the material’s ductility while maintaining hardness. The key to success lies in managing the temperature range and soak time during these stages.
Moreover, controlling the cooling rate after heating influences the formation of a microstructure with distinct characteristics, such as fine pearlite or martensite, depending on the process goals. This manipulation is essential for achieving high wear resistance in tools, bearings, and cutting instruments. For example, tools that require a hard, wear-resistant edge benefit from rapid quenching, while parts that need improved shock resistance can be tempered at lower temperatures to reduce brittleness.
Another critical application is in the production of carburized components, where a high-carbon surface layer is introduced to enhance surface hardness. By using specific heat treatment cycles, it is possible to create a gradient of hardness with a tough, low-carbon core and a hard outer layer, optimizing both durability and resistance to wear. Understanding how different phases interact under varying temperatures is integral to achieving the desired material properties efficiently.