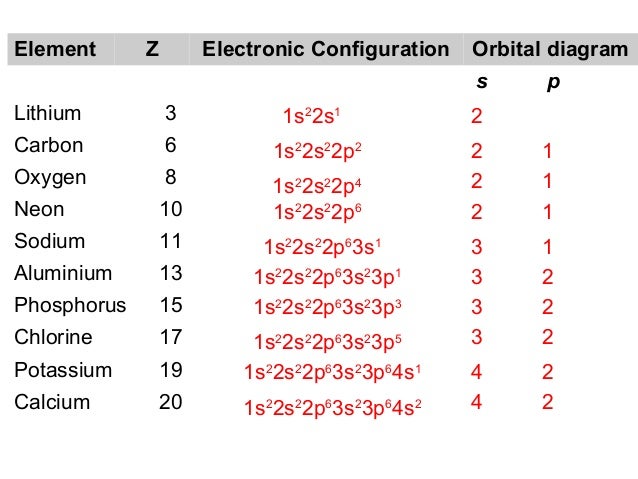
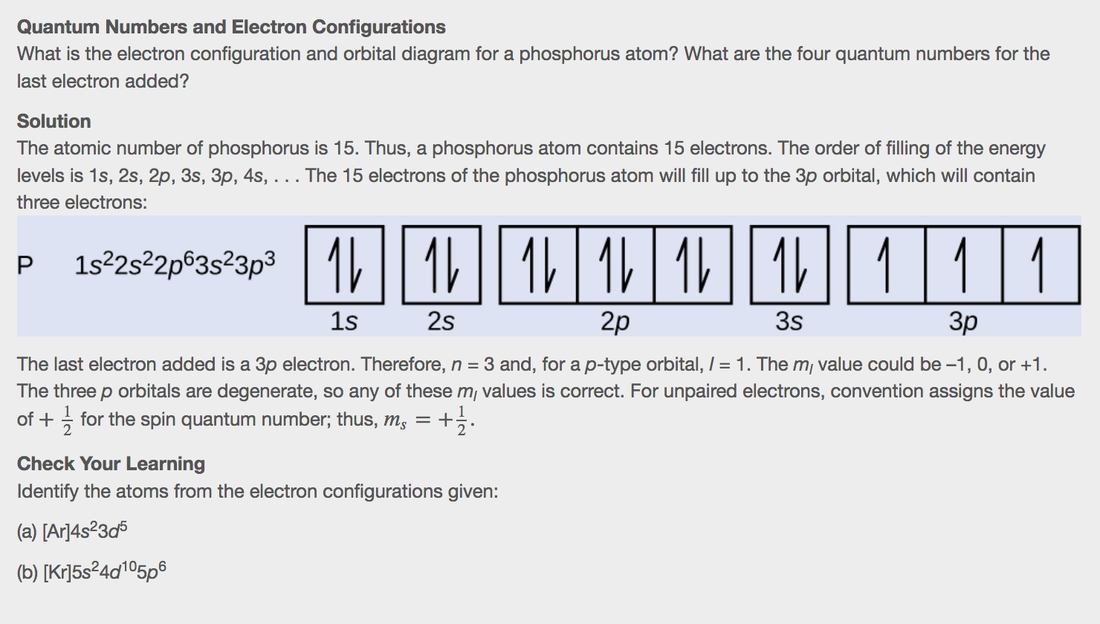
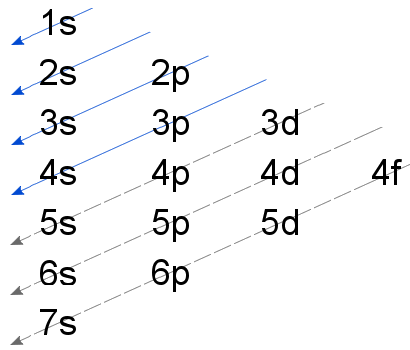
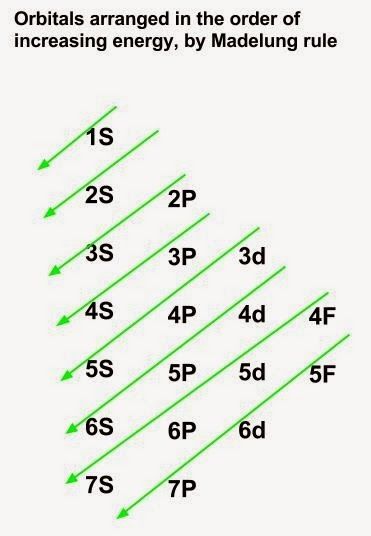
The aufbau principle states that in the ground state of an atom or ion, electrons fill atomic An example is the configuration 1s2 2s2 2p6 3s2 3p3 for the phosphorus atom, meaning that the 1s subshell has 2 electrons etc.
Aufbau is a German. Electron Configurations and Orbital Diagrams KEY.

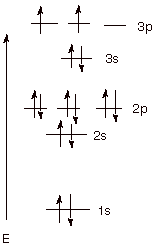
Draw orbital diagrams for the following elements: 1. phosphorus. ↑↓.
↑↓. ↑↓ ↑↓ ↑↓.
↑↓. ↑ ↑ ↑.
1s. First, he Aufbau principle requires that lower energy orbitals are filled with Since phosphorus in a third period element, the first (K) and second (L) shells are .
Phosphorus, P, is located in period 3, group 15 of the periodic table, and Therefore, the electron configuration of a neutral phosphorus atom. Electron Configuration Notation: shows the arrangment of electrons around the nucleus of an atom.
– helps chemist understanding how elements form chemical.Orbital diagrams show the distribution of electrons in the electron shells and subshells of an atom. A few principles and rules must be followed in this process.
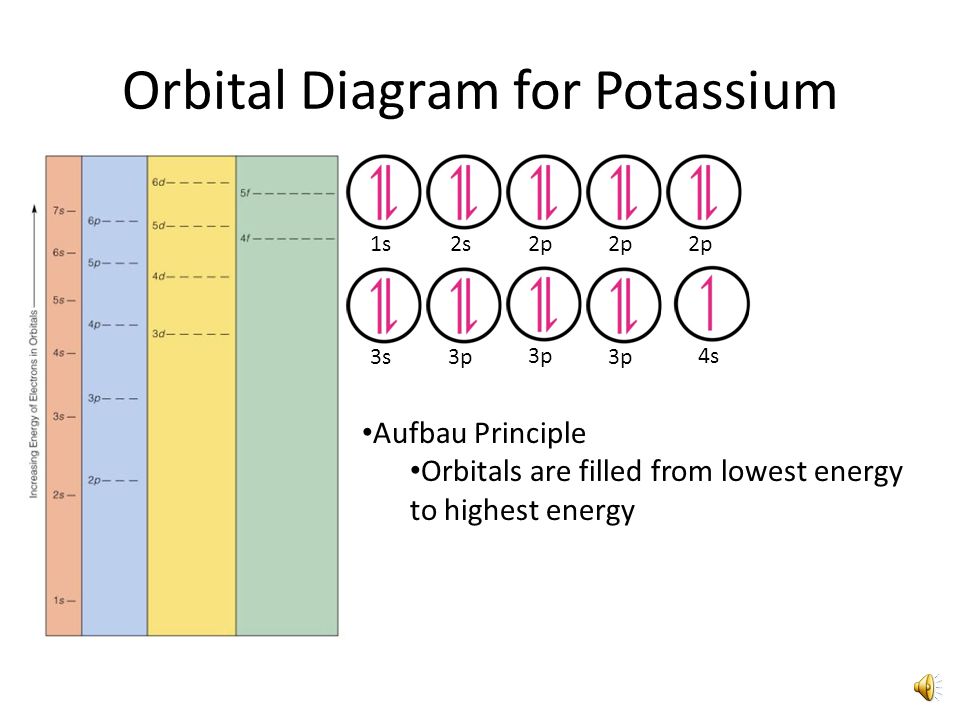
First, he Aufbau principle. What is the electron configuration and orbital diagram for a phosphorus atom?
What are the four quantum numbers for the last electron added? Solution. The atomic number of phosphorus is Thus, a phosphorus atom contains 15 electrons.
The order . The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3 s orbital, giving a 1 s 2 2 s 2 2 p 6 3 s 1 configuration.
Locate the nearest noble gas preceding phosphorus in the periodic table. Then subtract its number of electrons from those in phosphorus to obtain the number of valence electrons in phosphorus. Referring to Figure , draw an orbital diagram to represent those valence orbitals.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital.
The p orbital can hold up to six electrons.Electron configuration – WikipediaAufbau principle – Wikipedia