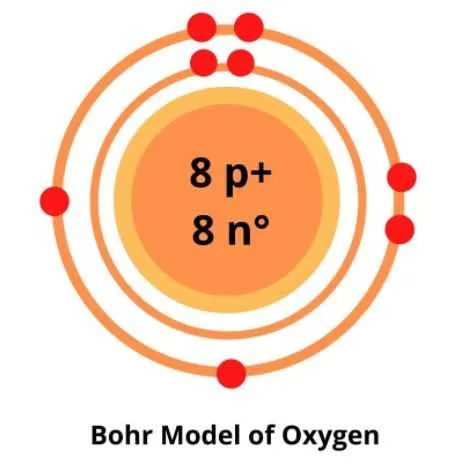
To understand the atomic structure of the eighth element in the periodic table, it’s essential to focus on the arrangement of its subatomic particles. The nucleus contains 8 protons, surrounded by 8 neutrons, while the electrons are organized in specific energy levels around the nucleus. The first shell holds 2 electrons, and the second shell can accommodate the remaining 6 electrons. This setup explains the element’s chemical behavior and reactivity.
Electron configuration follows a 2, 6 pattern in this case, with the first two electrons in the innermost shell filling up the 1s orbital, and the other six occupying the second shell in the 2s and 2p orbitals. This configuration gives the element its characteristic valency, explaining its tendency to form bonds by accepting or sharing electrons.
The configuration of electrons in various shells directly influences the element’s physical and chemical properties. In particular, the placement of the electrons in the second shell, close to the nucleus, plays a significant role in the formation of covalent bonds and the interaction with other atoms, especially in molecular formation.
Atomic Model of Oxygen
The atomic structure of this element consists of 8 protons in the nucleus, with 8 electrons arranged in energy levels. The first shell holds 2 electrons, while the second shell contains 6 electrons. This configuration satisfies the stability requirements of the atom, ensuring the outer shell is nearly full but not complete.
In the first energy level, two electrons occupy the closest orbit to the nucleus. The second energy level, further from the nucleus, holds the remaining 6 electrons. The atom’s chemical reactivity is primarily influenced by the 6 electrons in the outermost shell, which are responsible for bonding with other atoms.
This arrangement leads to a typical valence of 2, with a tendency to form covalent bonds by sharing electrons with other atoms to achieve a more stable configuration. The atom’s ability to form bonds is fundamental to its role in molecular compounds such as water.
The inner orbitals, which are completely filled, contribute to the atom’s overall stability and do not usually participate in chemical reactions. The configuration of the 6 electrons in the second shell determines its electronegativity, making it highly effective in attracting electrons when bonding with other elements.
Understanding Electron Configuration in the Bohr Model
The atomic structure of this element can be described using a central nucleus surrounded by electrons. For a stable configuration, electrons fill shells starting from the innermost layer. This element has a total of 8 electrons, which are distributed in two energy levels: 2 electrons in the first shell and 6 electrons in the second shell.
To visualize this, think of the first shell as holding up to 2 electrons and the second shell as accommodating up to 8 electrons. Since the second shell of this atom holds 6 electrons, it is considered partially filled. The arrangement of electrons influences the atom’s reactivity and bonding capabilities, specifically its tendency to form covalent bonds by sharing electrons in the outermost shell.
In terms of quantum mechanics, the first shell is closest to the nucleus and has the lowest energy, while the second shell, further away, holds higher energy electrons. This configuration affects the chemical properties, including the formation of bonds with other atoms to achieve a more stable state.
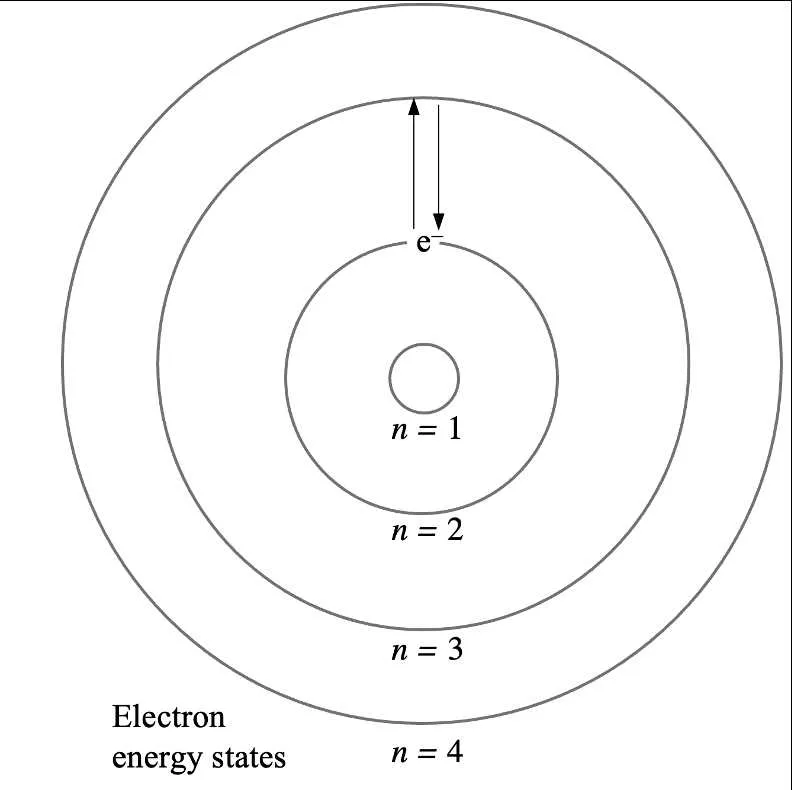
How to Visualize the Energy Levels of Oxygen in the Atomic Model
To effectively represent the energy levels of this element, start by focusing on its electron configuration. The atom contains 8 electrons, with 2 electrons in the first shell and 6 in the second. The first shell can hold a maximum of 2 electrons, while the second shell has a capacity of 8 electrons. Since oxygen has only 6 electrons in the second shell, this shell is not full.
Next, place the 2 electrons in the innermost shell. For the outer shell, position the remaining 6 electrons around the nucleus, distributed in a way that reflects their energy state–generally in two sets of 2 electrons in opposite directions, with the final pair occupying the remaining orbital positions.
Indicate the energy levels by drawing concentric circles around the nucleus. Label the first circle as the 1st shell (lowest energy), and the second circle as the 2nd shell (higher energy). These levels correspond to the principal quantum numbers (n = 1 for the first shell, n = 2 for the second shell). The first level is closest to the nucleus, meaning it has the lowest energy. The second level holds the remaining electrons and has a higher energy state.
To reflect the relative energy of these levels, consider the following: the energy required to add an electron to the outer shell is higher than that needed to add an electron to the inner shell. This distinction helps to understand the atom’s reactivity and bond formation potential. Use these principles to construct a precise visual representation that conveys the electron distribution clearly.
Practical Applications in Chemistry
The atomic model of a molecule with eight protons and electrons is invaluable for understanding the behavior of elements in various chemical reactions. By analyzing electron configurations and energy levels, this model allows precise predictions of reactivity, bonding, and molecular interactions. Below are key applications:
- Electron Configuration Analysis: Understanding the distribution of electrons helps predict how atoms will interact in chemical bonds, particularly covalent bonding where sharing of electrons occurs.
- Redox Reactions: This model aids in tracking electron transfer during oxidation-reduction reactions, essential for processes like respiration and combustion.
- Acid-Base Chemistry: The arrangement of electrons in an atom helps explain why certain compounds behave as acids or bases by analyzing their tendency to donate or accept electrons in aqueous solutions.
- Catalysis: In catalysis, understanding how electron configurations influence reaction mechanisms can optimize catalysts and reaction rates, as seen in industrial chemical processes.
- Materials Science: This model is used to design materials with specific properties, such as semiconductors, by understanding the behavior of electrons in different energy states.
- Spectroscopy: Knowledge of electron transitions aids in interpreting spectroscopic data, which is essential for identifying molecular structures and reaction pathways.
For chemists, mastery of this atomic model enables enhanced control over reactions, allowing for more efficient production and novel material development.