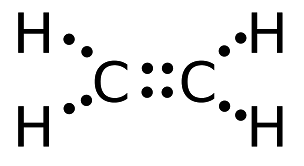
Draw the dot structure of C2H4.
Ask for details; Follow; Report. by IreneRoses Log in to add a comment.
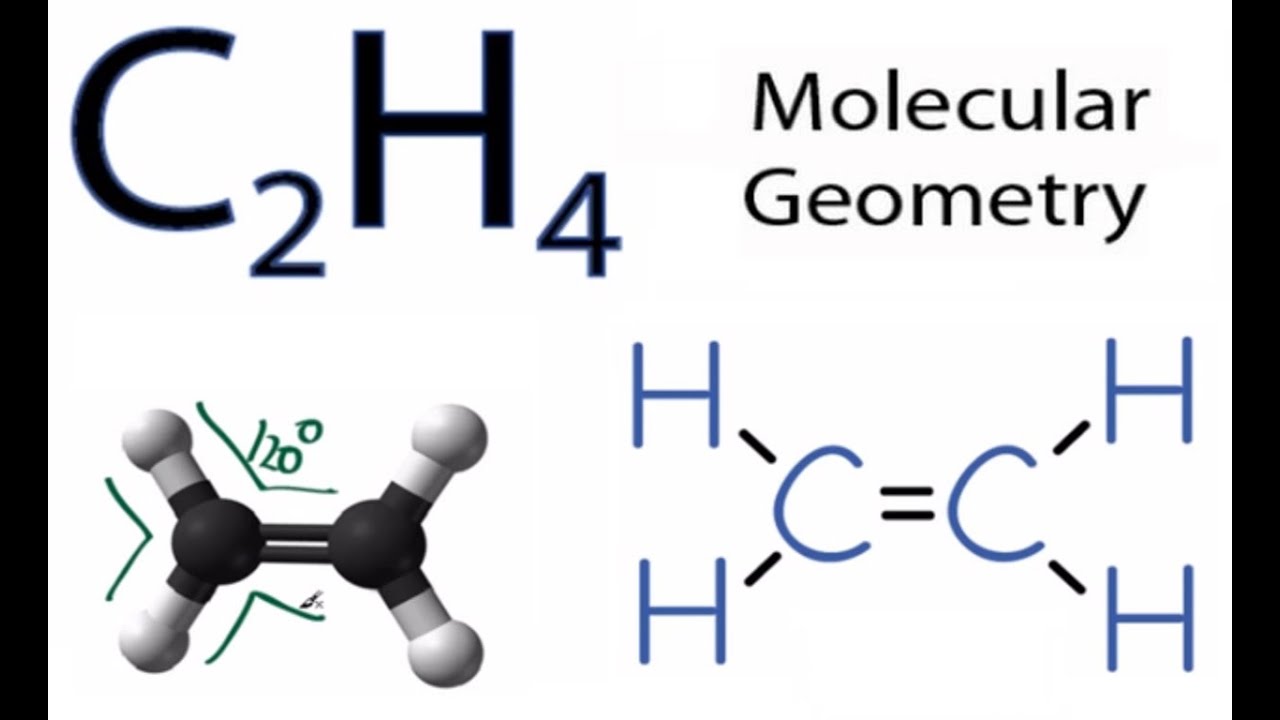
Lewis Structures for C2H4. Step-by-step tutorial for drawing the Lewis Structure for C2H4.
The Lewis structure of C2 H4, also known as ethene, has two carbons with a double bond between them. This means that the carbon atoms share 4 electrons.
Electron Dot Structure for ethane, C2H4. The key to understanding how to distribute the valence electrons is to recognize the need for a double.

Lewis Structures for C2H4. Step-by-step tutorial for drawing the Lewis Structure for C2H4.Drawing the Lewis Structure for C 2 H 4.
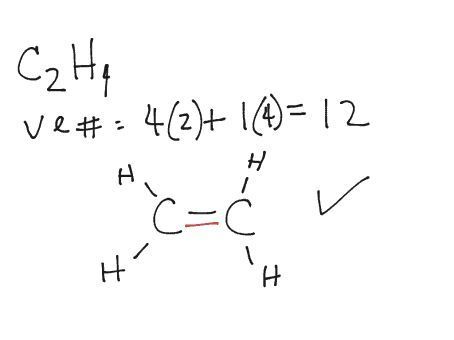
For C 2 H 4 you have a total of 12 total valence electrons.. Drawing the Lewis structure for C 2 H 4 (named ethene) requires the use of a double bond.
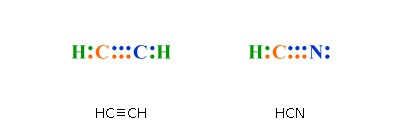
In a double bond two pairs of valence electrons are shared (for a total of four valence electrons). I would be lazy and look it up on the internet.
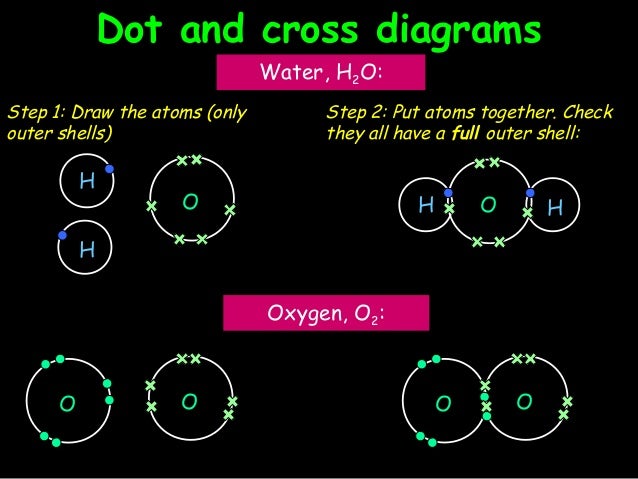
But seriously, you have an electron pair between the C and each of the H’s in the Lewis diagram a ala. Drawing the Lewis Structure for C 2 H 2 (Ethyne or Acetylene).
For C 2 H 2 you have a total of 10 valence electrons to work with.. In drawing the Lewis structure for C 2 H 2 (also called ethyne) you’ll find that you don’t have enough valence electrons available to satisfy the octet for each element (if you use only single bonds).
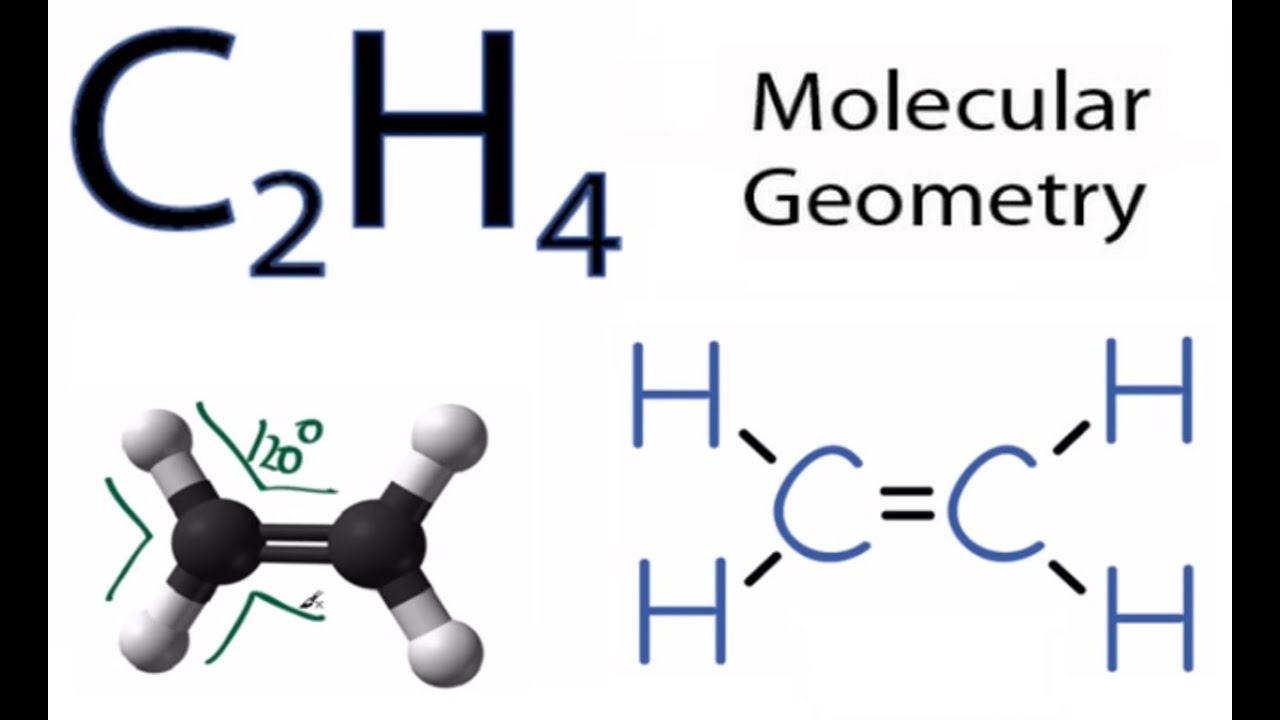
The solution is to share three pairs of valence electrons and. Chemical Bonding: Lewis Dot Structure for C2H4 (6 of 6) Watch the video of Dr. B.
drawing the Lewis dot structure for C 2 H 4 (ethene) and answer the questions below. Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons.
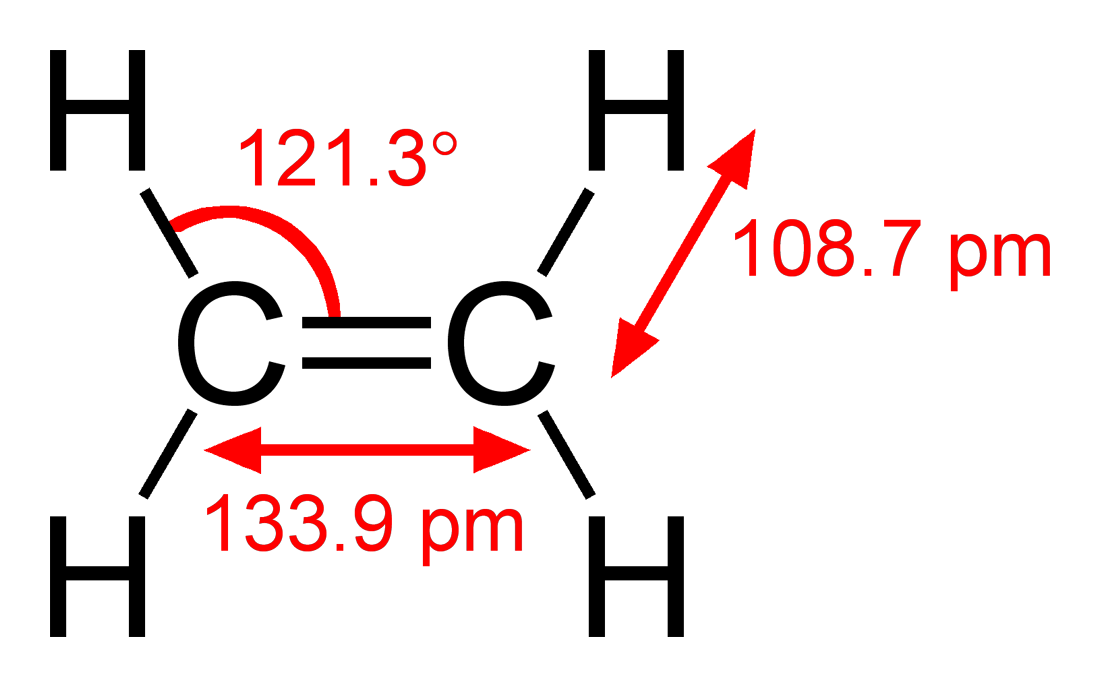
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula C 2 H 4 or H 2 C=CH schematron.org is a colorless flammable gas with a faint “sweet and musky” odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds)..

Ethylene is widely used in the chemical industry, and its worldwide production (over million tonnes in ) exceeds that of any other organic.Lewis structure of C2H4:BiochemhelpLewis Structure for C2H4