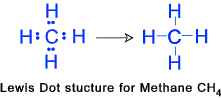
Lewis structures help us to determine how atoms within a molecule are connected, i.e. which atom connects to which, and using how many bonds.
In addition. Lewis Structures for CH4.
Step-by-step tutorial for drawing the Lewis Structure for CH4. Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule.
C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane. We also have This info can then be used to determine the Lewis Dot Structure.
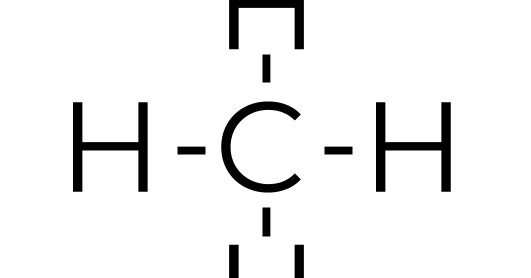
Find an answer to your question draw the electron dot structure of CH4.Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons..
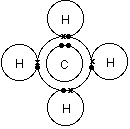
Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org’s one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
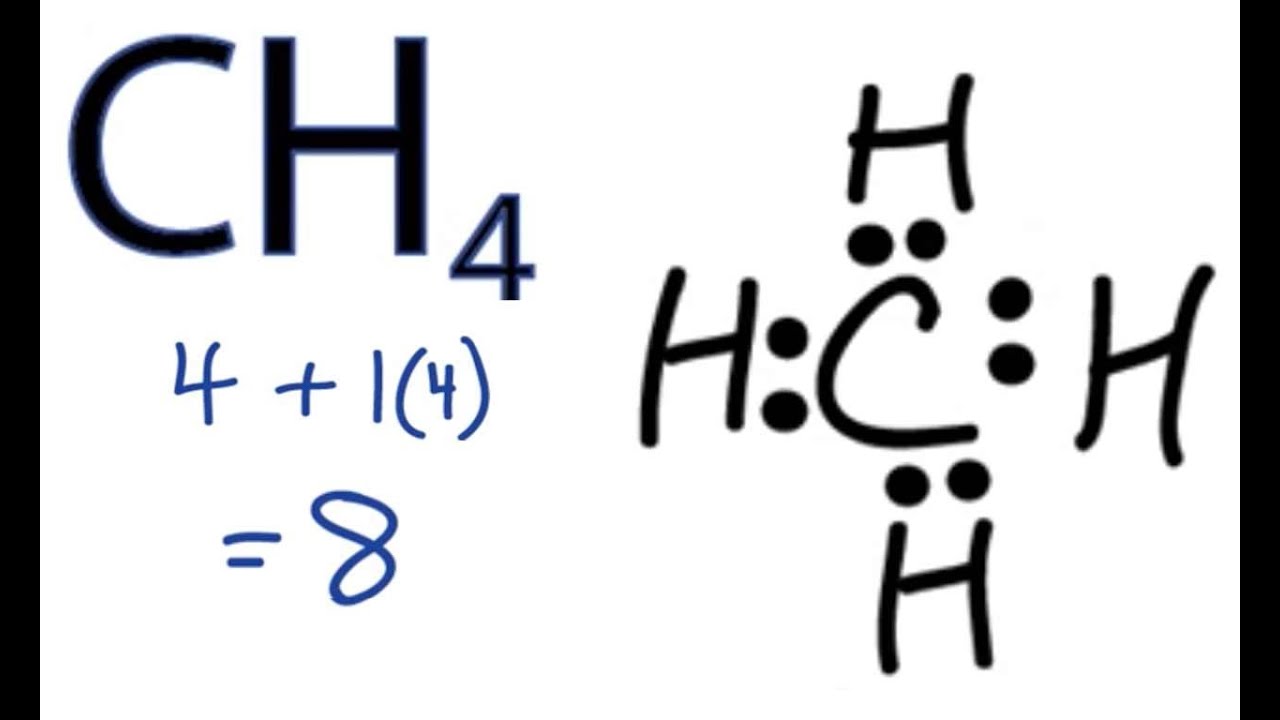
The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to .
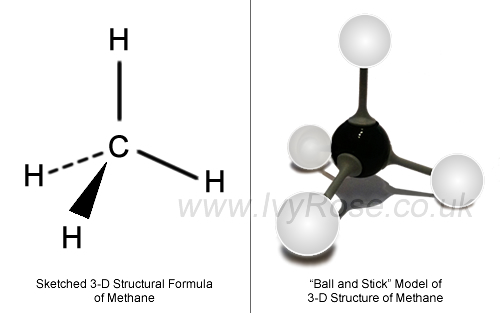
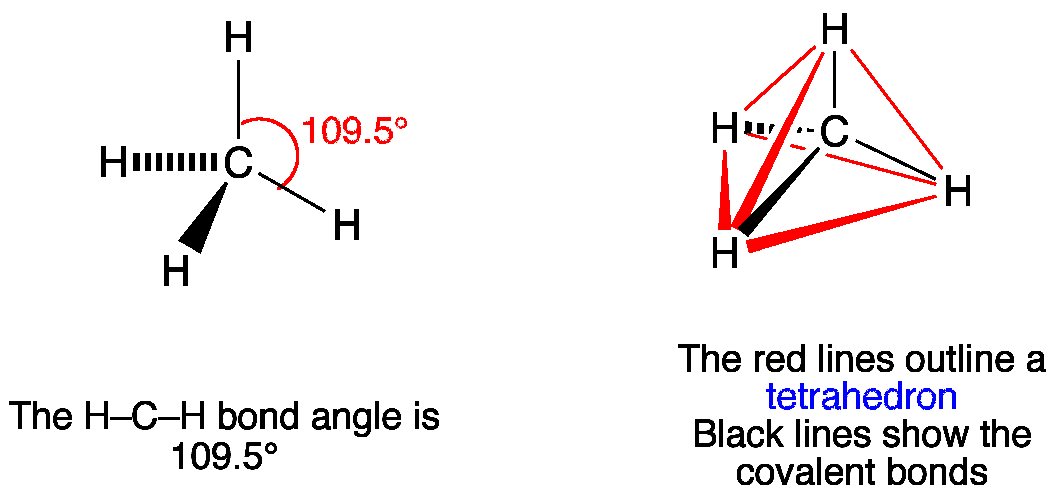
First draw the Lewis dot structure: Electron geometry: tetrahedral. Hybridization: sp 3 Then draw the 3D molecular structure using VSEPR rules: Decision: The molecular geometry of methane – CH 4 – is tetrahedral with symmetric charge distribution on the central atom. Lewis dot structure of CH 4.
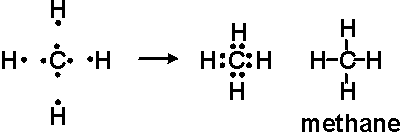
Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1×4=4.
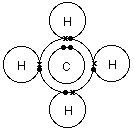
Total=8. Put carbon in center and arrange hydrogen atoms on the schematron.orge electrons between carbon and hydrogen atoms.
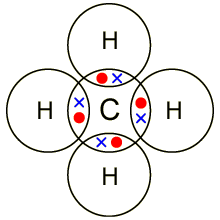
Lewis structures, also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.The Lewis Dot Structure for CH4 – MakeTheBrainHappyLewis structure – Wikipedia