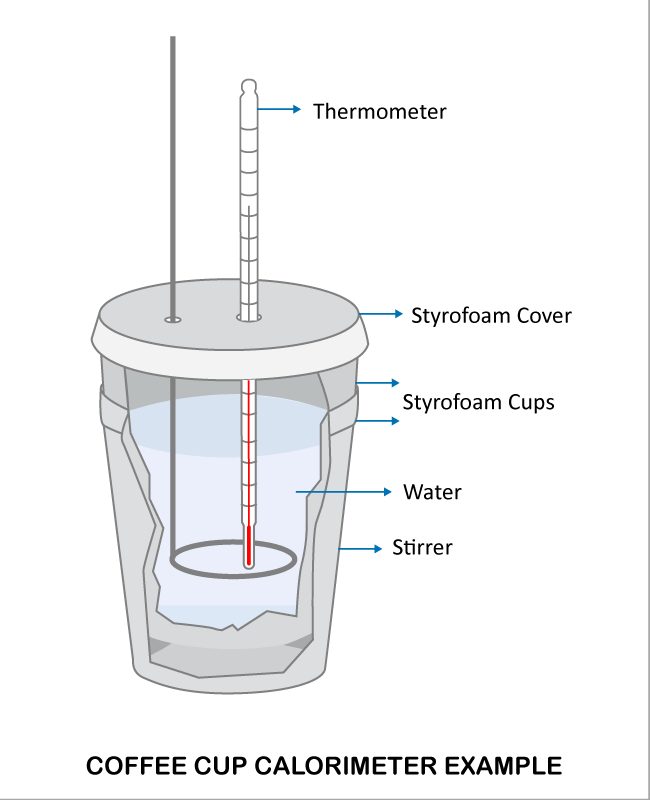
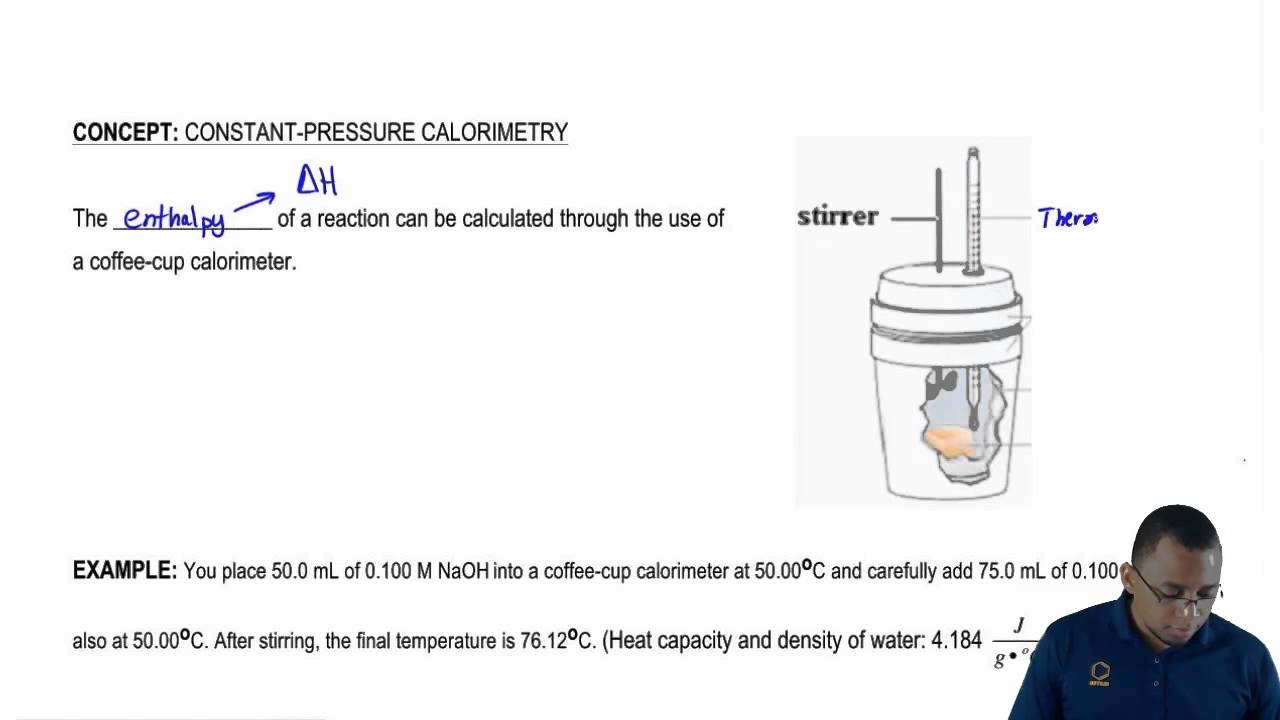
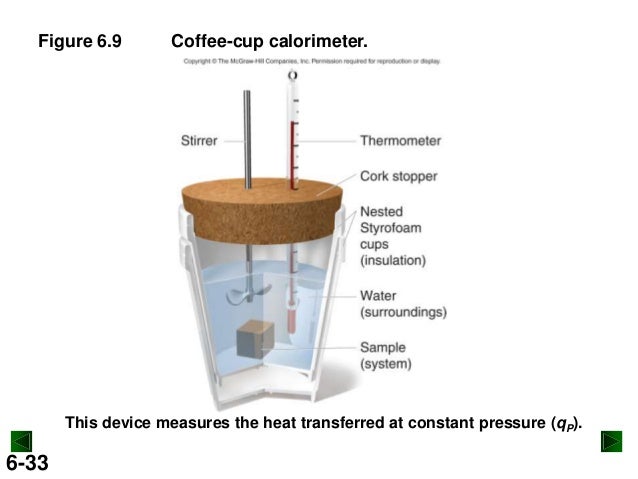
A coffee cup calorimeter is a constant pressure calorimeter.
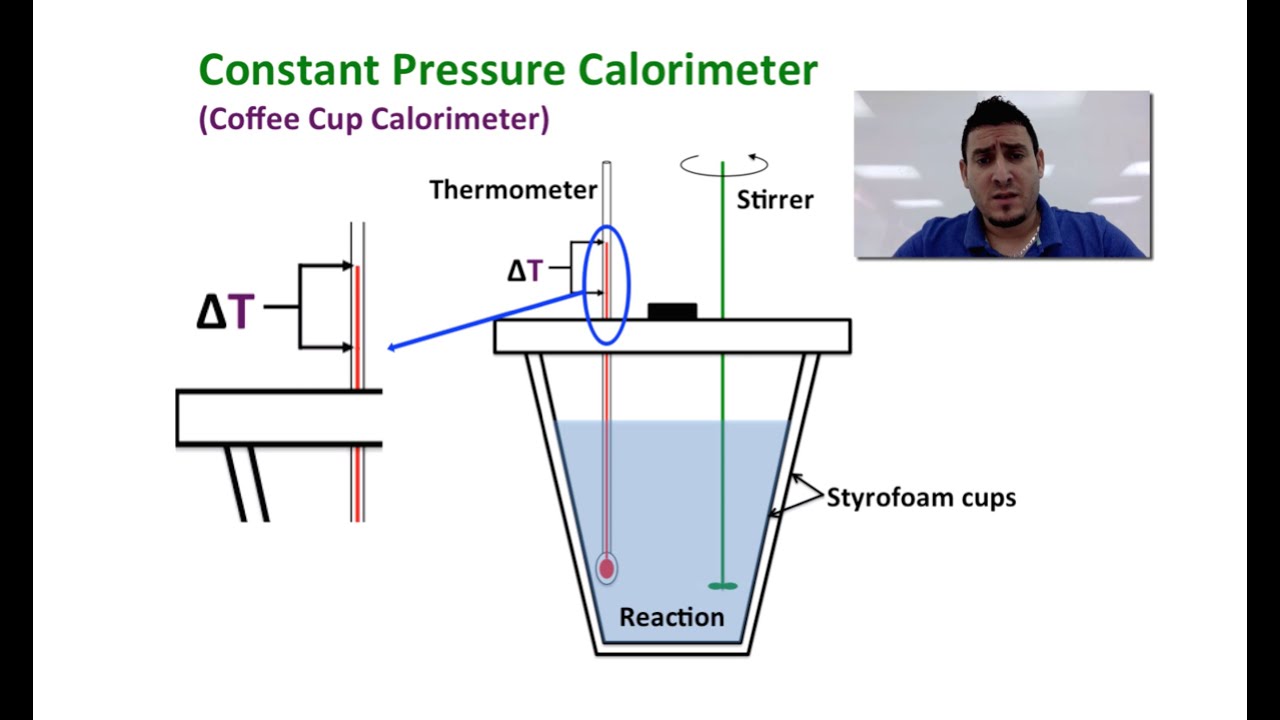
As such, the heat that is measured in such a device is equivalent to the change in enthalpy. A coffee. In a Coffee-Cup Calorimeter, reactants are mixed in a solution, usually water, and measure the temperature change. Since styrofoam acts as a.
One kind of calorimeter, known as a coffee cup calorimeter, is shown at left. Coffee cup calorimeters are usually used to measure changes that take place in.
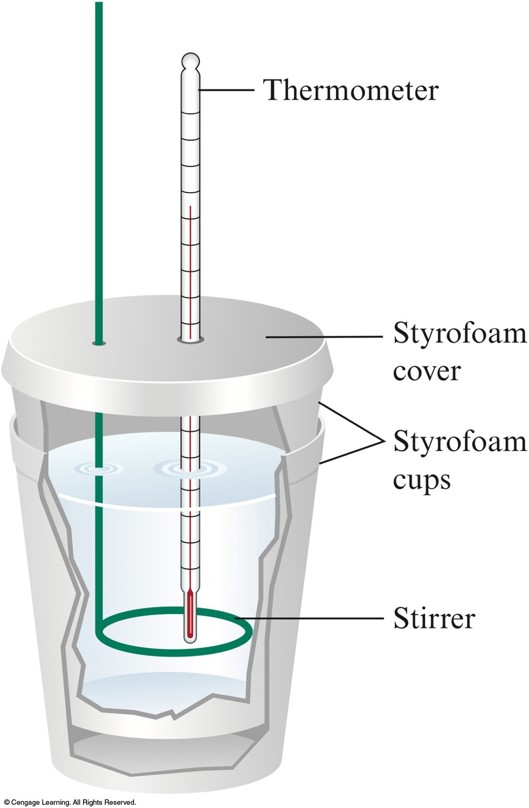
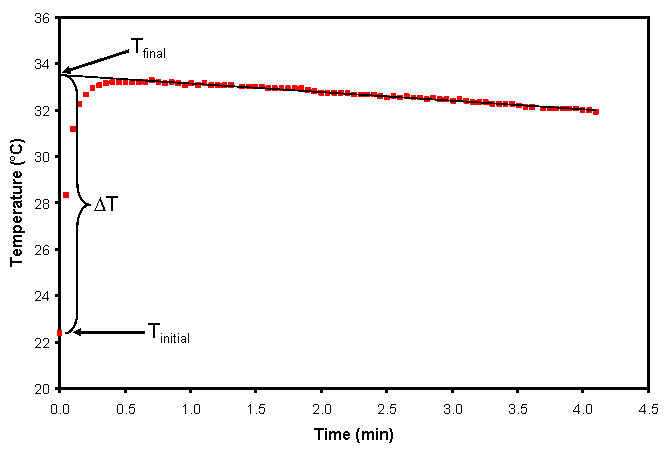
The ubiquitous and imperfectly insulated coffee cup calorimeter is .. Figure 4. Exothermic reaction temperature and heat loss correction.
Calorimetry
Rather, it is because the calorimeter used in high school science labs is more commonly referred to as a Styrofoam cup. It is a coffee cup calorimeter – usually.Basically a calorimeter is a device used to measure either specific heat capacity or the amount of energy produced or absorbed in a chemical reaction.
It functions by putting a said liquid of known amount into a thermally insulated container, then. Coffee Cup Calorimeter When a chemical reaction occurs in the coffee cup, the heat generated by the reaction will be absorbed by the water in the cup.
Because Styrofoam is a good insulator, nearly all the thermal energy from the reaction will stay in the cup and not . COFFEE CUP CALORIMETER Gary L.
Coffee Cup Calorimeter
Bertrand. close.
A coffee cup calorimeter is simply a styrofoam cup (or maybe one cup inside another) to provide insulation when materials are mixed inside of it. A styrofoam cover and a sensitive thermometer complete the apparatus.
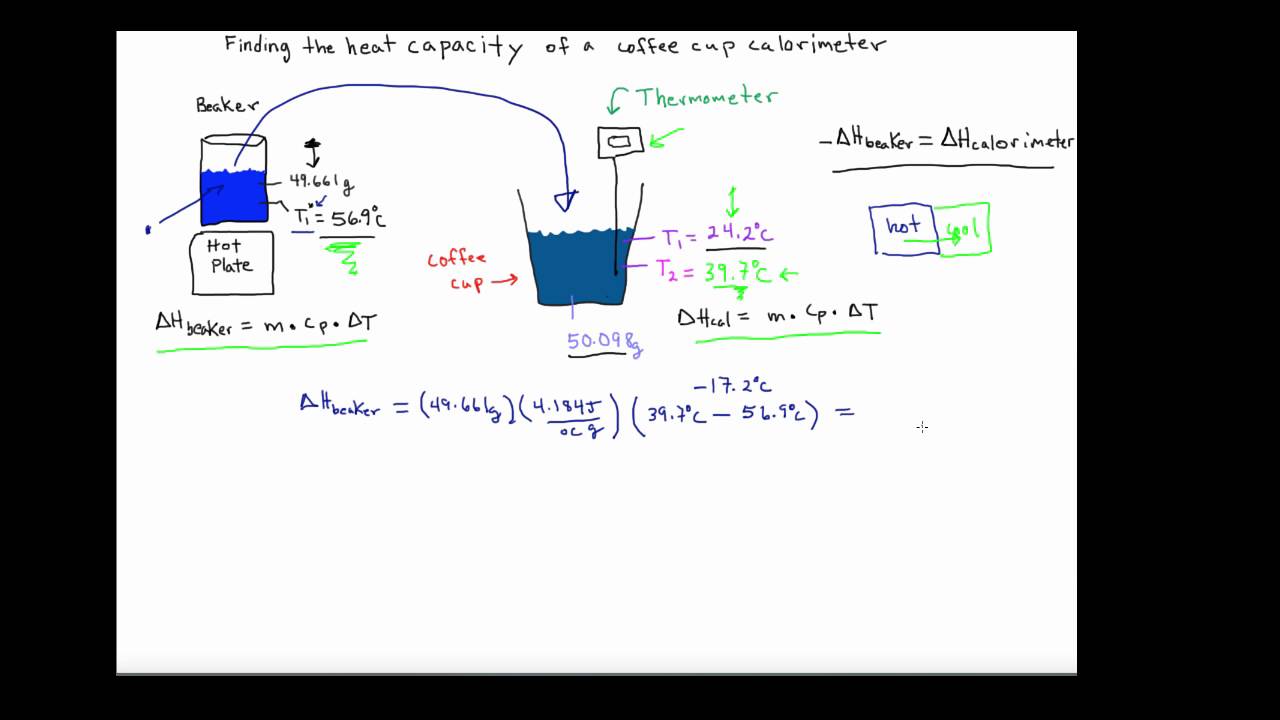
Coffee Cup Calorimeter Problem – General Chemistry
Chem. Final.

STUDY. PLAY.
Which statement describes how a basic coffee cup calorimeter works? It uses the mass and specific heat of water along with a thermometer to measure the gain or loss of energy when a substance is added.
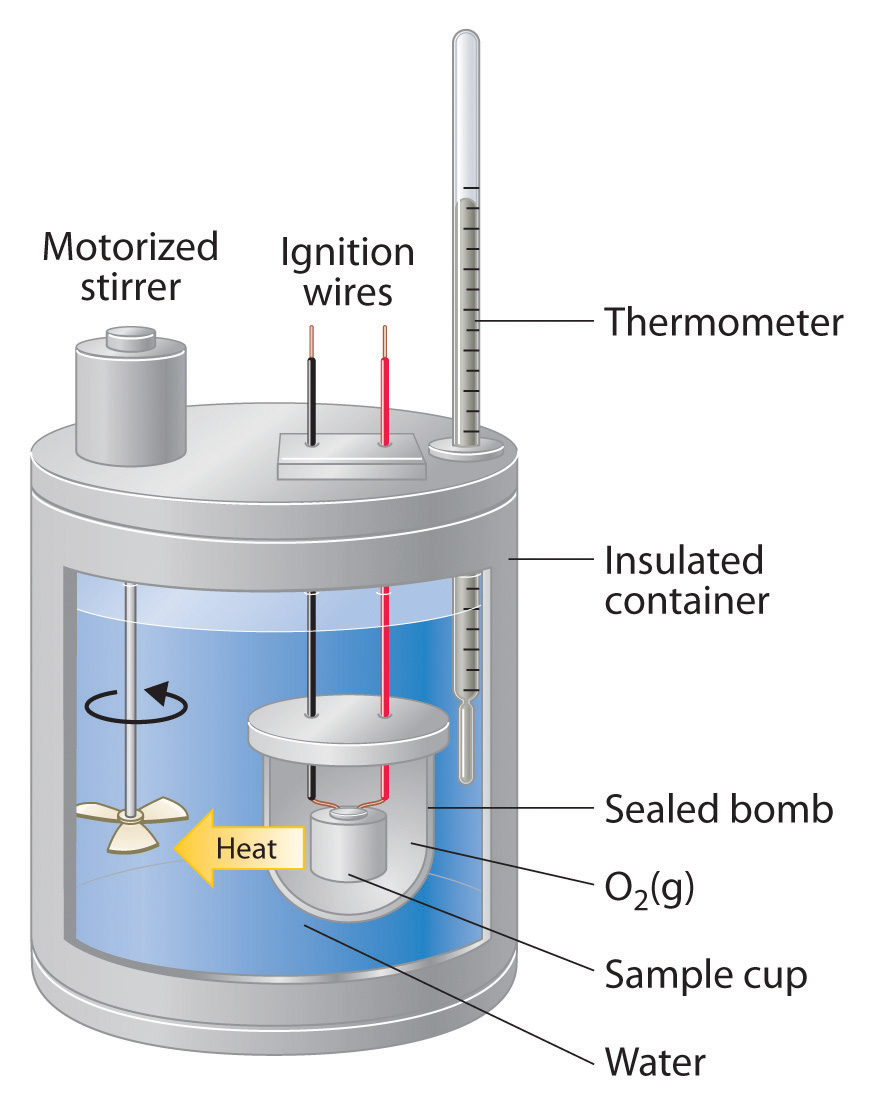
Which diagram is a bomb calorimeter? b.
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat produced by the reaction is absorbed by the solution, which increases its temperature.Calorimeters and CalorimetryCalorimeter – Wikipedia