(The name crystal field arose because the theory was first developed to .
Figure Crystal-field splitting in a series of octahedral chromium(III) complexes. .
the d orbitals in the appropriate crystal-field splitting diagram in each case. Similar Questions.
Chemistry. Based on crystal field theory, which of the following metal ions will not be colored when placed in an octahedral crystal field?.
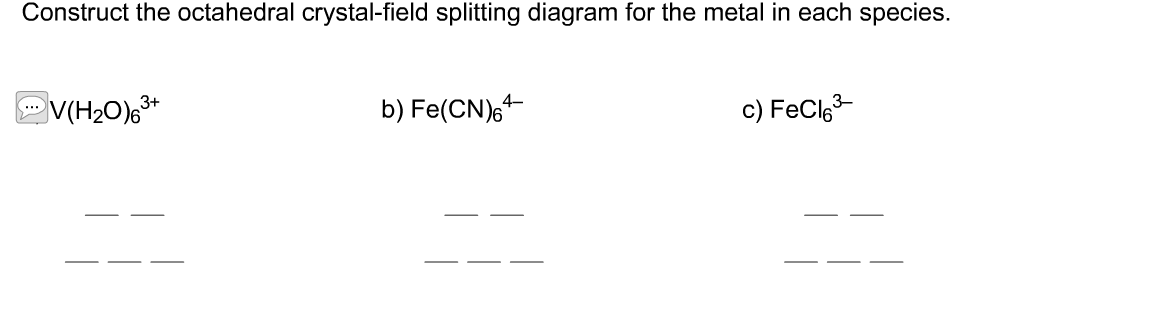
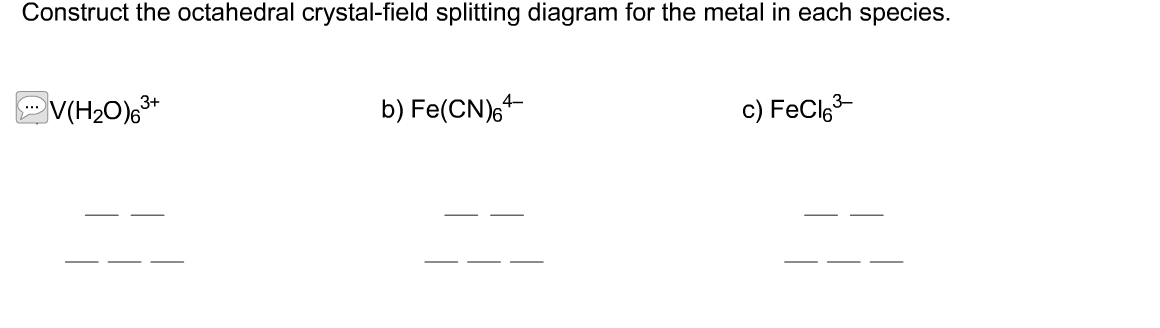
Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 – Mn(H2O)62+.
A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
Solved: Construct the octahedral crystal-field splitting diagram for the metal in each species. a) [math]V(H_2O)_6^{3+}[/math] a) [math]Co(CN)_6^{3-}[/math] a) .(The energy gap,, is sometimes referred to as the crystal-field splitting energy.
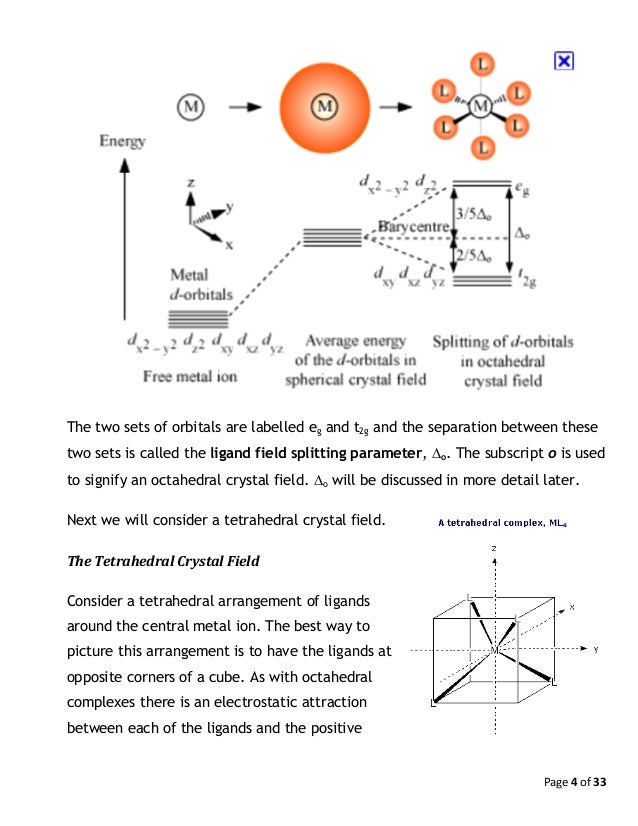
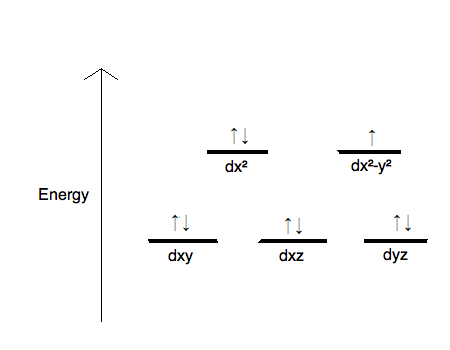
Figure Energies of the d orbitals in an octahedral crystal field. Let’s examine how the crystal-field model accounts for the observed colors in transition-metal complexes.
Construct the octahedral crystal-field splitting diagram for the metal in each species. Nov 14, · Basically, the question is referring to the compound K3[Fe(C2O4)3].
It asks what is the electron configuration in this comound, I got it to be d5. Fe in the compound is Fe(III) so 23 electrons -> d5. It then asks how many unpaired electrons and asks Status: Resolved.

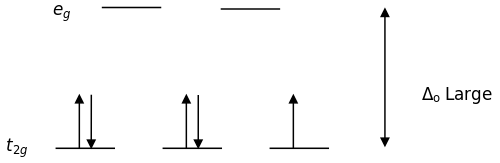
In an octahedral field, where the d-orbitals are split into two sets (e g and t 2g), a high spin d 5 configuration would result in all orbitals containing 1 unpaired electron (see below). A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm.
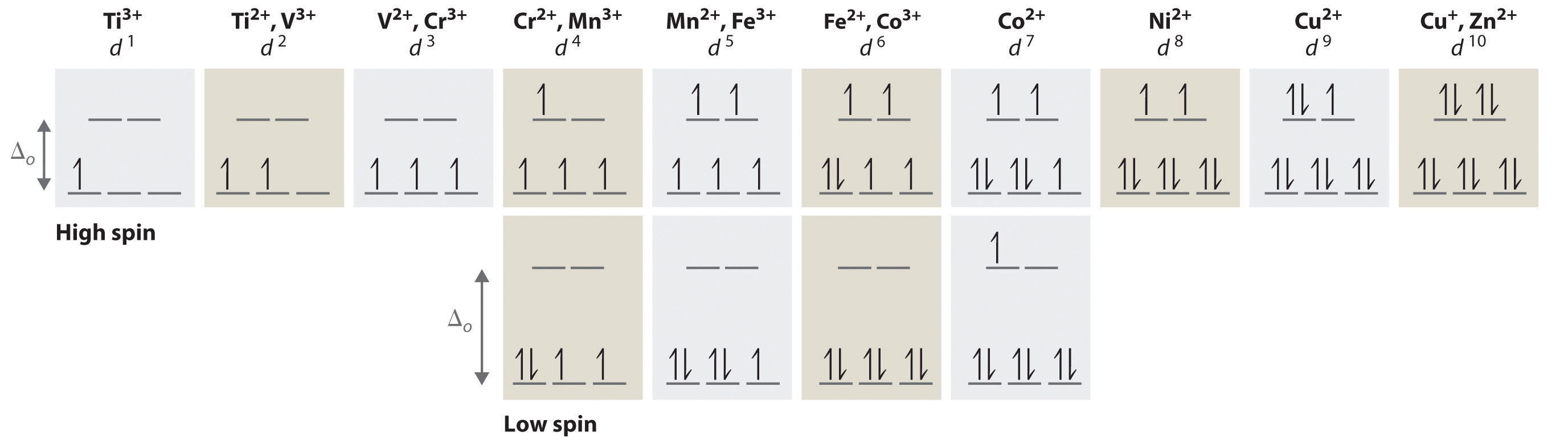
a) Calculate the crystal-field splitting energy, Δ, in kJ/mol. asked by Jematormal91 on May 31, ; Chemistry terms. Match the terms with the correct definitions.
1. ligand 2. coordination compound 3.Chemistry: The Central Science, Chapter 24, Section 5Crystal field theory – Wikipedia