The effects of pH on the form in which an element in a given oxidation state exists in natural waters can be summarized with predominance diagrams such as. Potential-pH diagrams are also called Pourbaix diagrams after the name of their Thus, Pourbaix diagrams introduce the concept of the following three states of .
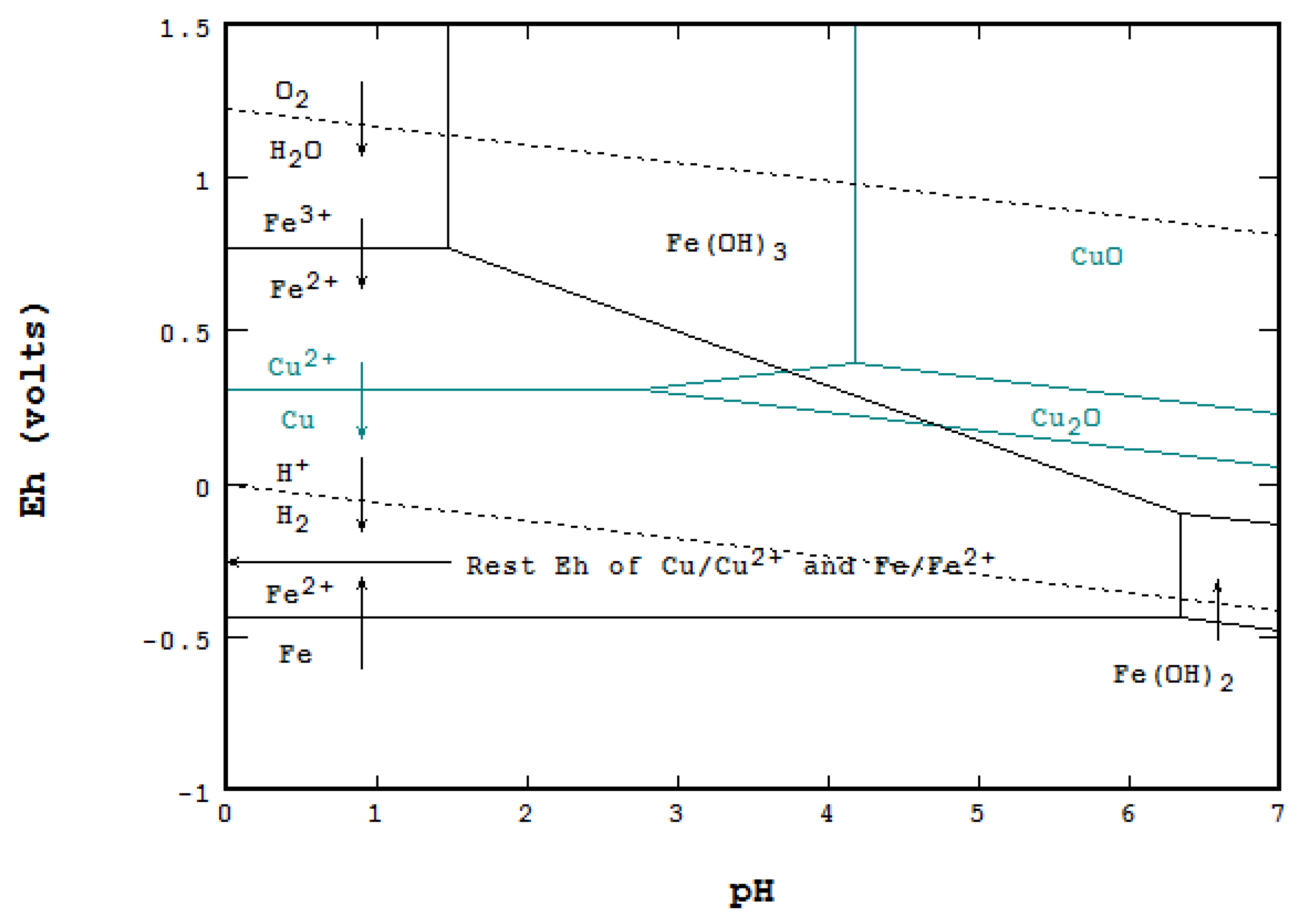
ABSTRACT Pourbaix diagrams (electrode potential-pH diagrams) for Cu-Br?- H2O systems at 25°C were developed in g/L and g/L (M and M). Potential-pH diagrams are also called Pourbaix diagrams after the name of their Thus, Pourbaix diagrams introduce the concept of the following three states of . The effects of pH on the form in which an element in a given oxidation state exists in natural waters can be summarized with predominance diagrams such as.In electrochemistry, a Pourbaix diagram, also known as a potential/pH diagram, E H-pH diagram or a pE/pH diagram, maps out possible stable (equilibrium) phases of an aqueous electrochemical system.
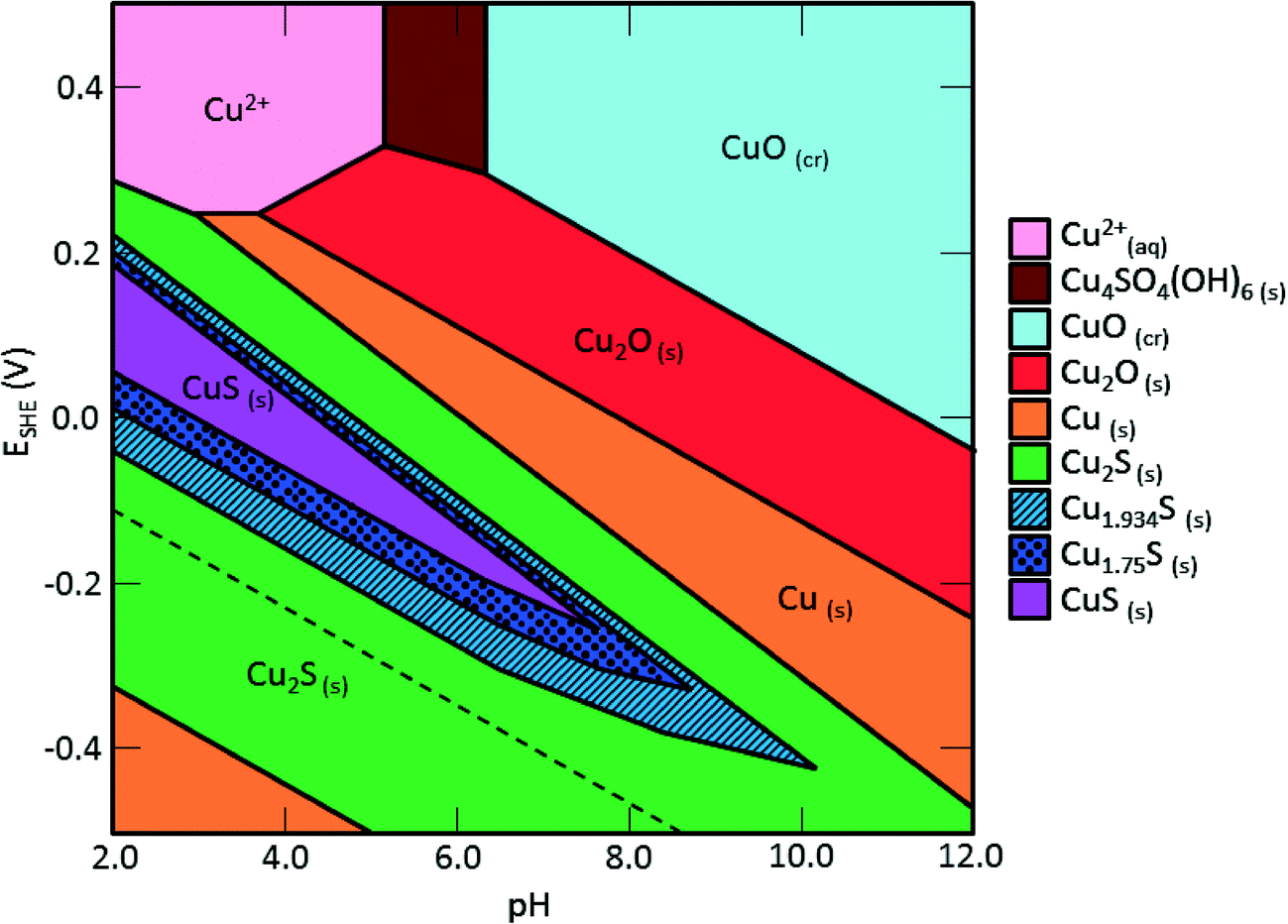
Predominant ion boundaries are represented by lines. These diagrams are available for over 70 different metals.
Pourbaix diagrams have several uses, including in corrosion studies. A Pourbaix diagram is also known as a potential/pH diagram, equilibrium diagram, EH-pH diagram, a pE/pH diagram on an E-pH diagram. thermodynamic equilibrium diagrams (E vs.

pH), called Pourbaix diagrams. A vast amount of data may be presented simply and concisely in Pourbaix diagrams.
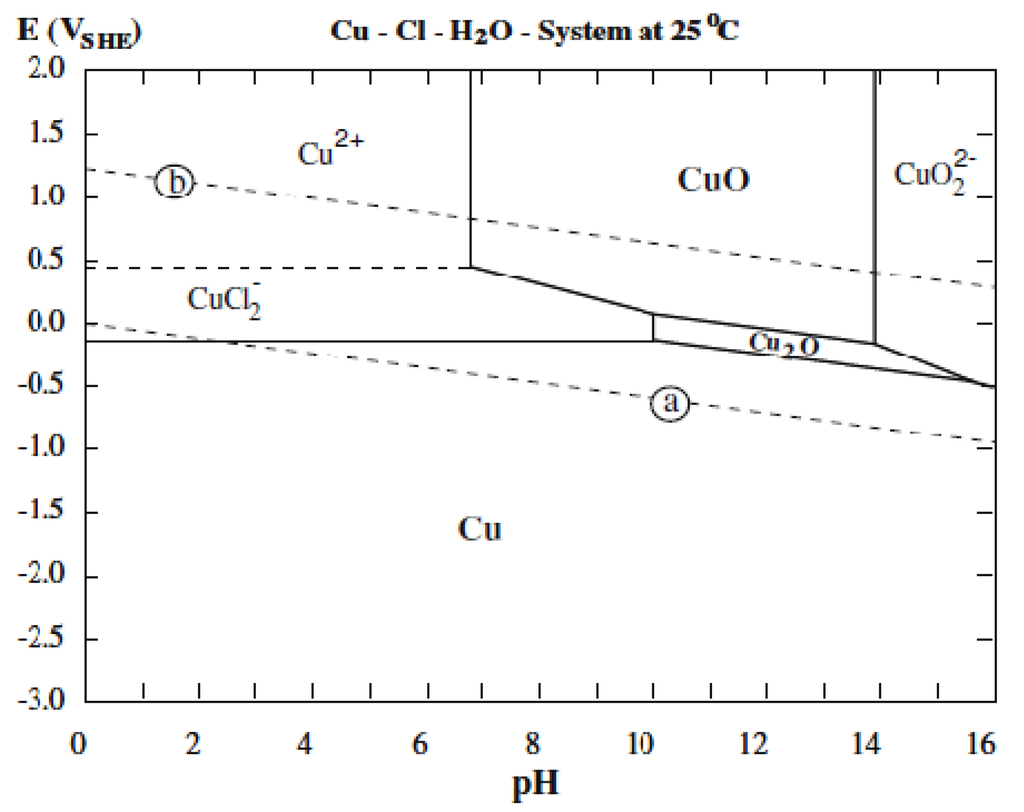
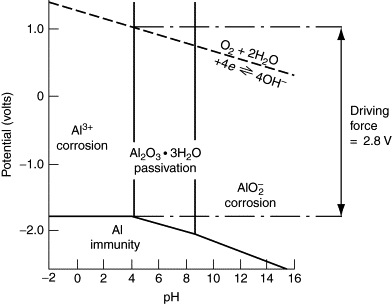
When the advantages and limitations of such diagrams are understood, valuable inferences may be made regarding corrosion phenomena. Pourbaix of Aluminum.
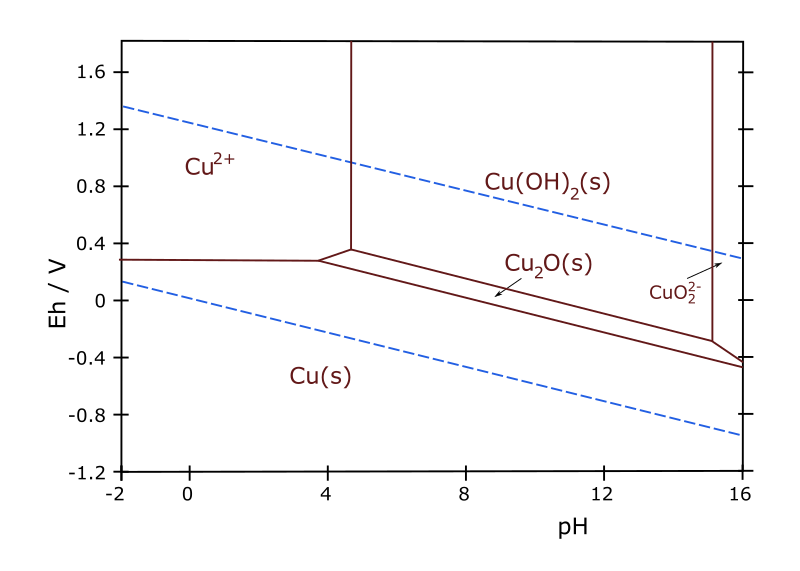
The pourbaix diagram of aluminum,indicates that hydrargilite Al. 2. O.
H. 2.
O, is the stable phase between about pH 4 and 9, indeed this film is considered to be responsible for successful use of aluminum in many structural applications. This. 2. Pourbaix diagrams are only one certain class of the phase diagrams, which gives a handy description of electrode potential v.s.
pH. There are other dimensions of parameters that are not shown on the Pourbaix diagrams.
For example, the activity of reactants other than H +, temperature, etc. The Pourbaix diagram is a projection of the.Copper Pourbaix Diagram | Physics ForumsCopper Pourbaix Diagram | Physics Forums