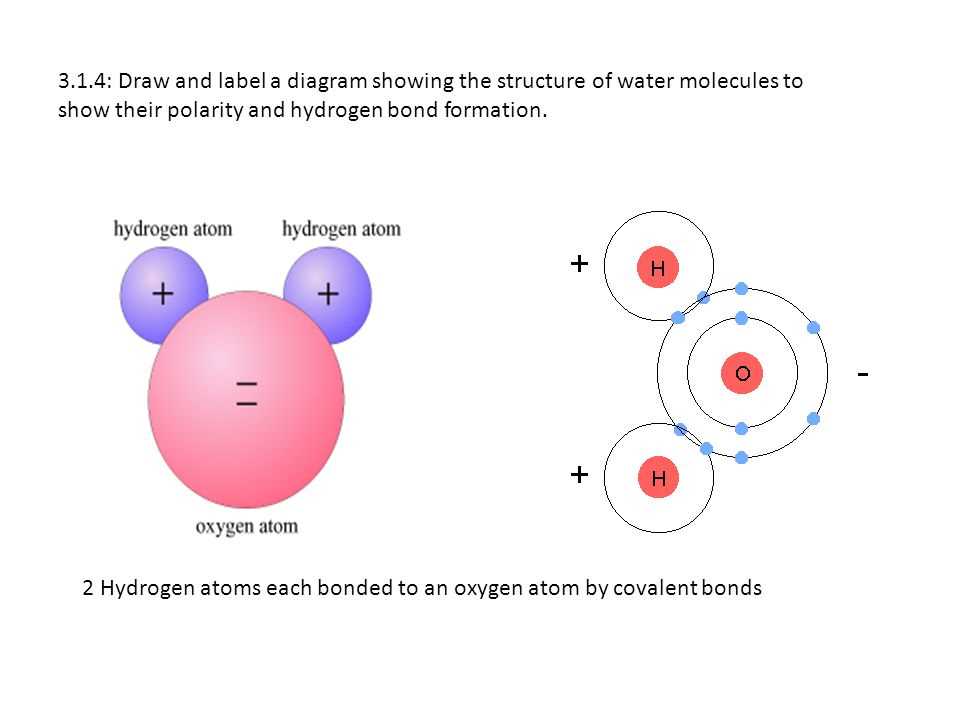
An atom is the basic unit of matter, consisting of a nucleus, which contains positively charged protons and uncharged neutrons, surrounded by negatively charged electrons. Understanding the structure of an atom is crucial to understanding the behavior and properties of matter.
This diagram of an atom with labels provides a visual representation of the various components of an atom. In the center of the diagram, you can see the nucleus, which is represented by a small circle. Inside the nucleus, there are protons, which are labeled with a “p+” and neutrons, labeled with an “n”. These particles are tightly packed together.
Surrounding the nucleus, you can see the energy levels or electron shells, represented by concentric circles. The first energy level, closest to the nucleus, can hold a maximum of two electrons, while the second energy level can hold a maximum of eight electrons. The diagram labels each electron with a small “e-“.
It’s important to note that the electrons do not move in a fixed orbit like planets around the sun. Instead, they exist in regions around the nucleus called orbitals, which are represented by shaded regions in the diagram. These orbitals describe the probability of finding an electron in a particular region of space.
Diagram of an Atom with Labels
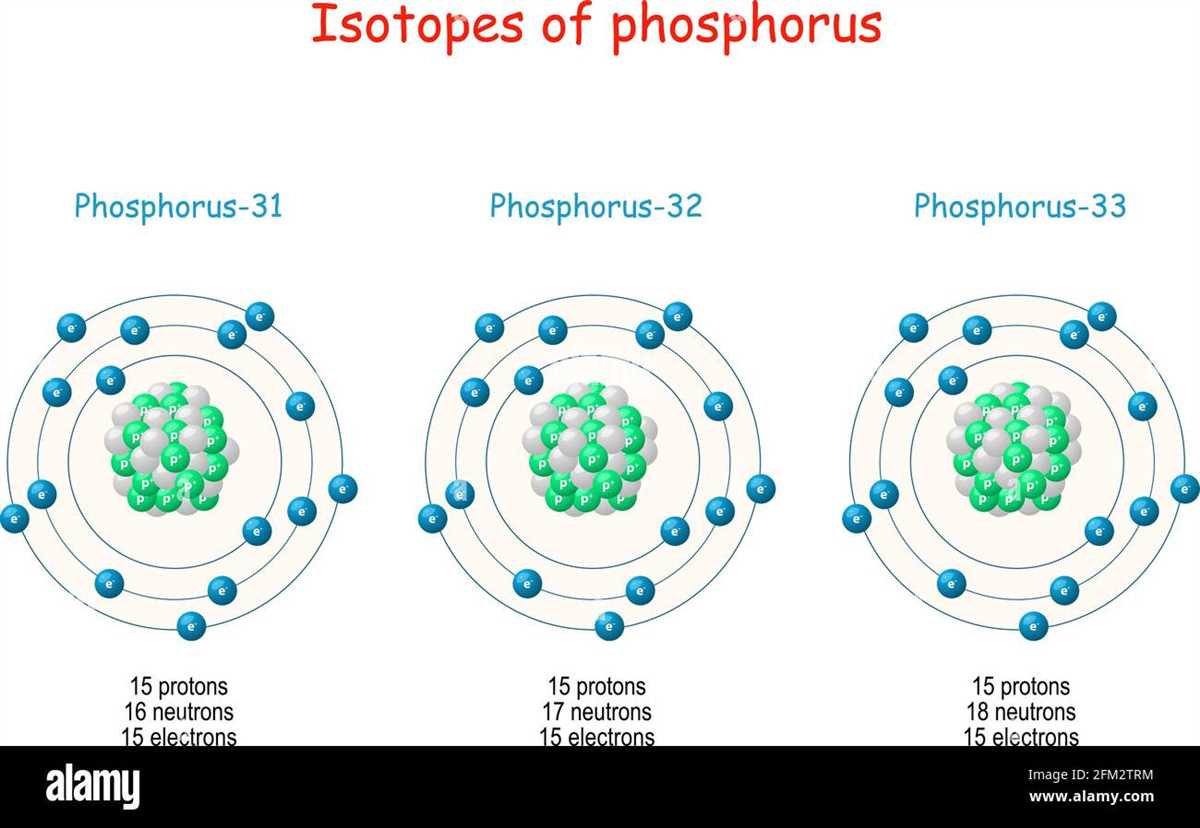
An atom is the basic building block of matter. It consists of three main components: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, which is the center of the atom. Electrons orbit around the nucleus in energy levels or shells.
Protons are positively charged particles found in the nucleus of an atom. They have a relative mass of 1 and a relative charge of +1. The number of protons in an atom determines its atomic number and defines the element. For example, all hydrogen atoms have one proton.
Neutrons are particles found in the nucleus of an atom. They have no charge and a relative mass of 1. Neutrons help stabilize the nucleus by counteracting the repulsive forces between protons, holding the nucleus together. The number of neutrons in an atom can vary, resulting in isotopes of the same element.
Electrons are negatively charged particles that orbit around the nucleus in specific energy levels or shells. They have a negligible mass compared to protons and neutrons. The number of electrons in an atom is equal to the number of protons, ensuring overall electrical neutrality. Electrons are organized in different shells, with the innermost shell closest to the nucleus and each subsequent shell at a higher energy level.
An atom can be represented in a diagram with labels to visually depict its structure. A typical diagram would show a circle in the center to represent the nucleus labeled “Nucleus.” Within the nucleus, smaller circles would indicate protons and neutrons, labeled accordingly. Orbiting around the nucleus would be smaller circles to represent electrons, with the appropriate number of circles in each energy level corresponding to the atomic number of the element. The diagram provides a clear visualization of the different components of an atom and their arrangement.
In conclusion, a diagram of an atom with labels helps illustrate the structure and composition of an atom. It highlights the presence of protons, neutrons, and electrons and their respective characteristics. This visual representation enhances understanding of atomic structure and the organization of particles within an atom.
What is an Atom?
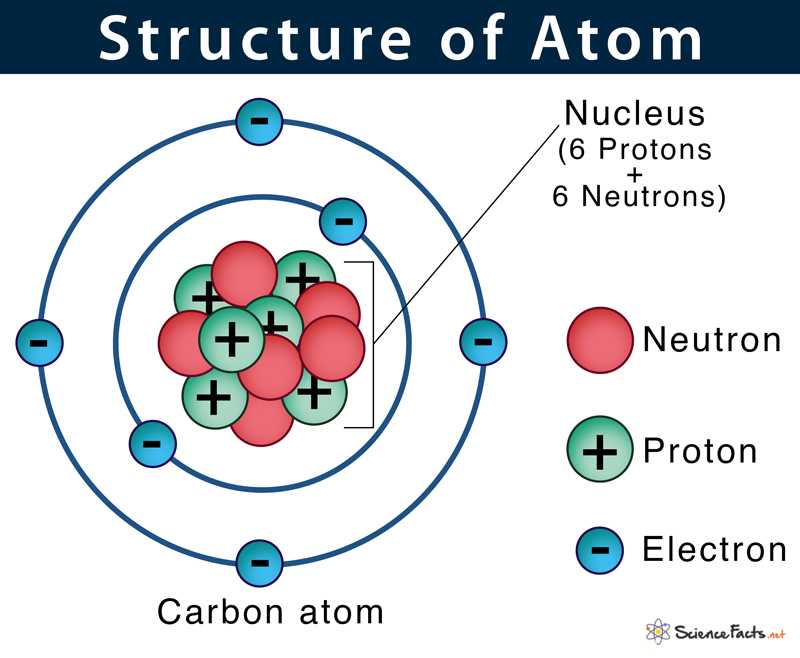
An atom is the smallest unit of a chemical element that retains the properties of that element. It consists of a nucleus, which contains positively charged protons and uncharged neutrons, surrounded by negatively charged electrons. The protons and neutrons make up the majority of the mass of an atom, while the electrons occupy the space outside the nucleus.
The nucleus of an atom is held together by the strong nuclear force, which overpowers the electrostatic repulsion between the positively charged protons. The number of protons in the nucleus is known as the atomic number, which determines the element’s identity. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon.
Protons are positively charged particles located in the nucleus of an atom. They carry a charge of +1 elementary charge and have a mass slightly less than that of a neutron. Protons are responsible for determining the element’s atomic number and play a role in chemical reactions and nuclear stability.
Neutrons are uncharged particles located in the nucleus of an atom. They carry no electrical charge and have a mass slightly greater than that of a proton. Neutrons contribute to the atomic mass of an element and help stabilize the nucleus by balancing the repulsive forces between protons.
Electrons are negatively charged particles that orbit the nucleus of an atom in energy levels or shells. They are much smaller and lighter than protons and neutrons. Electrons are involved in chemical bonding, determining the chemical reactivity of an element, and are responsible for the conductivity of materials.
In summary, an atom is the basic building block of matter and is composed of protons, neutrons, and electrons. Protons and neutrons make up the nucleus, while electrons occupy the space outside the nucleus. The arrangement and organization of these atomic particles determine the properties and behavior of different elements.
Structure of an Atom
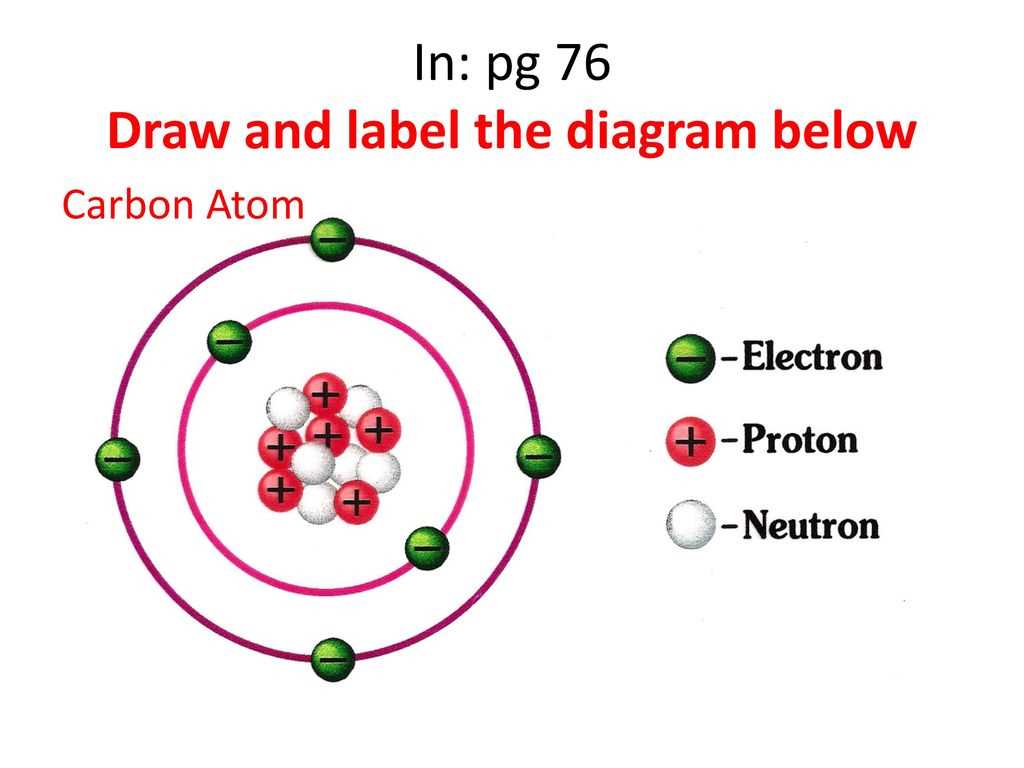
An atom is the basic building block of matter. It is composed of subatomic particles, including protons, neutrons, and electrons.
The nucleus of an atom is located at its center and is made up of protons and neutrons. Protons have a positive charge, while neutrons have no charge. The number of protons in an atom determines its atomic number, which defines the element. For example, hydrogen has one proton, while carbon has six.
The electrons of an atom orbit around the nucleus in specific energy levels called electron shells. Electrons have a negative charge and are attracted to the positively charged protons in the nucleus. The first electron shell can hold up to two electrons, while the second shell can hold up to eight electrons. The outermost shell is known as the valence shell and determines the chemical properties of the atom.
The distribution of electrons within the electron shells is represented by electron configuration diagrams, such as the Bohr model or the electron cloud model. These diagrams show the different energy levels and the number of electrons in each level.
The overall structure of an atom can be visualized using a diagram. This diagram typically includes the nucleus in the center with protons and neutrons labeled inside. The electron shells are depicted as circles surrounding the nucleus, with the number of electrons in each shell indicated. The diagram helps to illustrate the organization and distribution of subatomic particles within an atom.
Key terms:
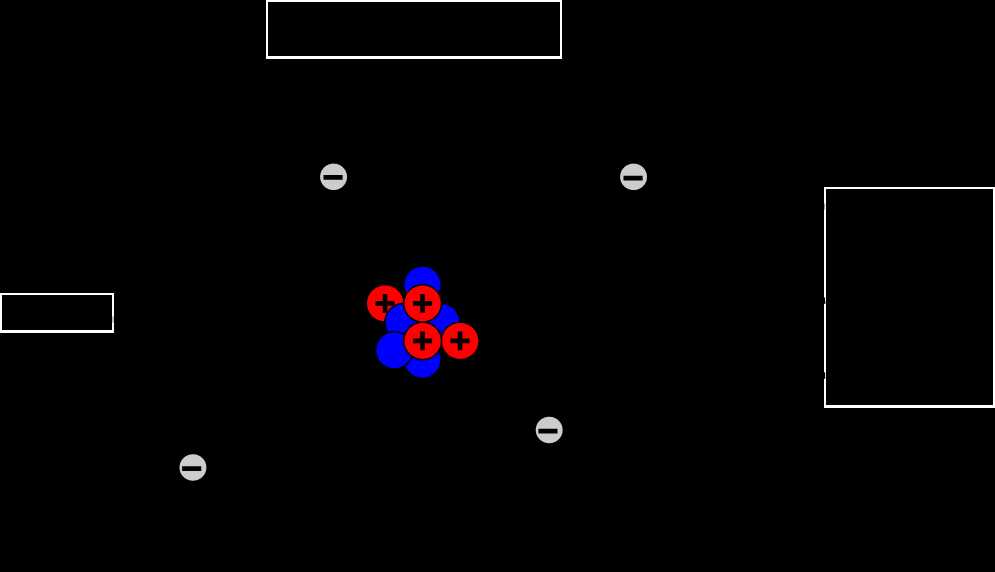
- Atom
- Subatomic particles
- Nucleus
- Protons
- Neutrons
- Electrons
- Atomic number
- Electron shells
- Valence shell
- Electron configuration
- Bohr model
- Electron cloud model
- Diagram
The Components of an Atom
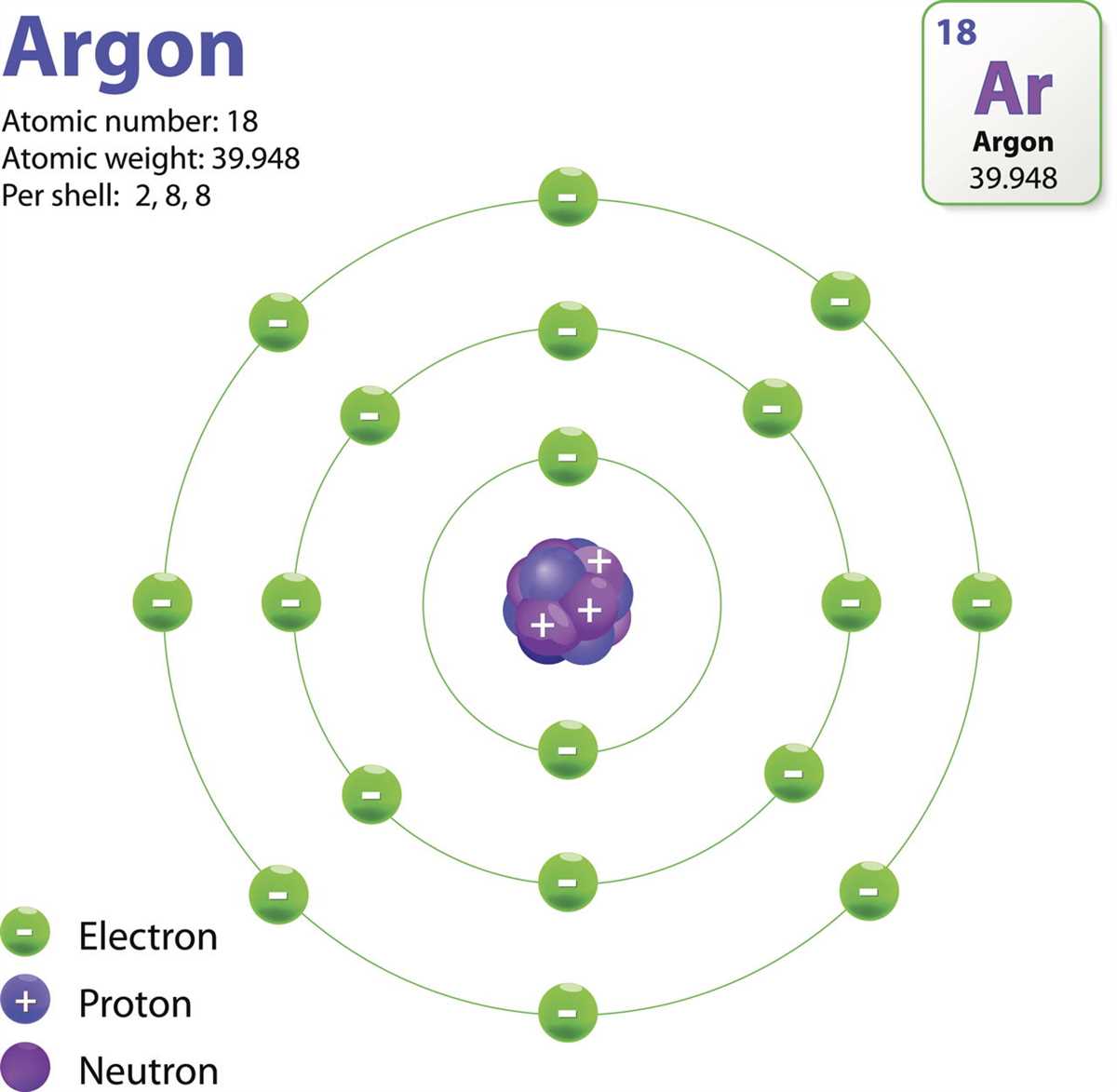
An atom is the basic building block of matter. It consists of several components, each playing a specific role in determining the properties and behavior of the atom.
1. Nucleus: The nucleus is the central part of the atom and contains most of its mass. It is made up of two types of subatomic particles: protons and neutrons. Protons have a positive charge, while neutrons have no charge. These particles are held together by the strong nuclear force, which is responsible for the stability of the nucleus.
2. Electrons: Electrons orbit the nucleus in energy levels or shells. They have a negative charge and are much smaller and lighter than protons and neutrons. Electrons are responsible for the chemical behavior of atoms, as they participate in chemical reactions and determine an atom’s ability to bond with other atoms.
3. Protons: Protons are positively charged particles found in the nucleus. They have a mass of approximately 1 atomic mass unit (amu) and a charge of +1. The number of protons in an atom determines its atomic number and defines the type of element it is.
4. Neutrons: Neutrons are neutral particles found in the nucleus. They have a mass similar to protons (approximately 1 amu) but no charge. The number of neutrons in an atom can vary, resulting in isotopes of the same element with different atomic masses.
5. Energy Levels: Electrons occupy specific energy levels or shells around the nucleus. The energy levels are represented by quantum numbers, and each level can hold a limited number of electrons. The innermost shell can hold up to 2 electrons, while the subsequent shells can hold up to 8 electrons.
6. Atomic Number: The atomic number of an atom is determined by the number of protons it contains. It defines the type and identity of the element. Elements are ordered in the periodic table based on their atomic numbers in ascending order.
7. Atomic Mass: The atomic mass of an atom is determined by the combined mass of its protons and neutrons. It is usually expressed in atomic mass units (amu). As electrons have negligible mass, they do not significantly contribute to the atomic mass of an atom.
In summary, atoms consist of a nucleus, which contains protons and neutrons, surrounded by electrons in energy levels. The number of protons determines the atomic number, while the combined mass of protons and neutrons gives the atomic mass. Electrons determine an atom’s chemical behavior. Understanding the components of an atom is crucial in understanding the properties and behavior of different elements and their interactions in various chemical reactions.
Electron Cloud
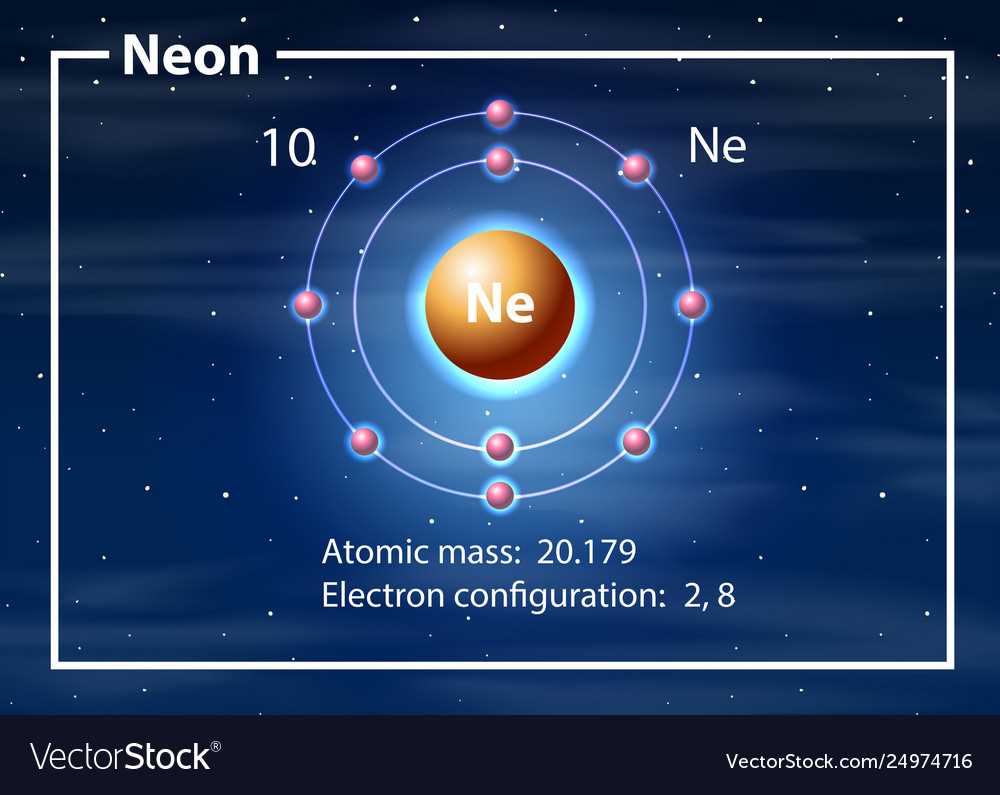
The electron cloud refers to the region surrounding the nucleus of an atom where electrons are most likely to be found. Unlike the planetary model of the atom, which depicts electrons as moving in fixed orbits, the electron cloud represents the probabilistic nature of electron behavior.
Quantum mechanics describes electrons as having both wave-like and particle-like properties. According to the Heisenberg uncertainty principle, it is impossible to simultaneously know the exact position and momentum of an electron. Instead, the electron’s location is described by a probability distribution, or wave function, which determines the likelihood of finding an electron at a given position.
The electron cloud can be visualized as a three-dimensional cloud surrounding the nucleus. The shape and size of the cloud depend on the energy level and orbital of the electron. Electrons with higher energy levels are found in larger, more diffuse clouds, while electrons with lower energy levels are found in smaller, more concentrated clouds.
Electron shells are regions within the electron cloud that contain electrons with similar energy levels. The first shell, closest to the nucleus, can hold up to two electrons, while the second shell can hold up to eight electrons. The electron configuration of an atom determines its chemical properties and reactivity.
- Valence electrons are the electrons in the outermost shell of an atom. These electrons are involved in chemical bonding and determine the atom’s ability to form chemical compounds.
- Electron density is a measure of the probability of finding an electron within a given volume of space. It is often represented by a contour plot, where higher density regions are represented by darker shading.
In summary, the electron cloud is the region surrounding an atom’s nucleus where electrons are most likely to be found. It is described by a probabilistic wave function and can be visualized as a three-dimensional cloud. The electron cloud plays a crucial role in determining the chemical properties and reactivity of atoms.
Atomic Nucleus
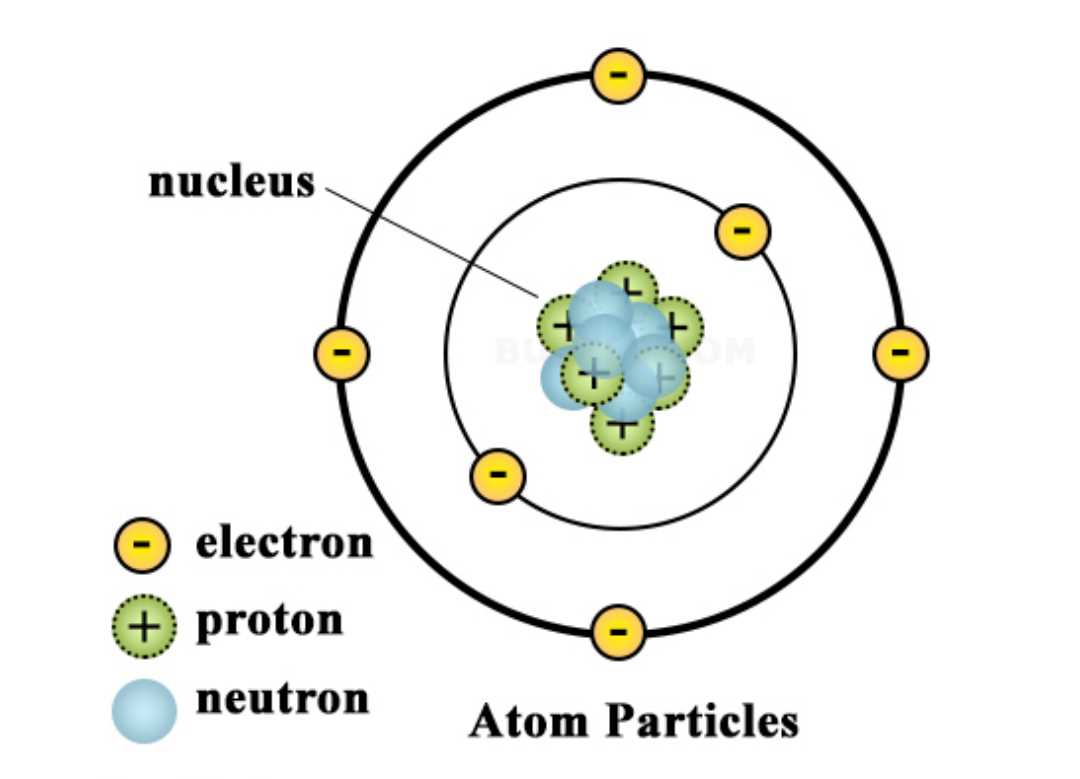
The atomic nucleus is the central part of an atom, consisting of protons and neutrons. It is often represented as a dense, positively charged core at the center of an atom. The nucleus accounts for almost all of the mass of an atom, even though it occupies only a tiny fraction of the total volume.
The protons in the nucleus carry a positive charge, while the neutrons are electrically neutral. Together, they help to stabilize the atom and define its properties. The number of protons in the nucleus determines the element’s atomic number, which is unique to each element. The total number of protons and neutrons in the nucleus is called the mass number.
Key Features of the Atomic Nucleus:
- The nucleus is small compared to the overall size of an atom.
- It contains most of the atom’s mass and virtually all of its positive charge.
- The protons in the nucleus repel each other due to their positive charges, but the strong nuclear force holds the nucleus together.
- The number of protons in the nucleus determines the element’s identity.
Understanding the structure and properties of the atomic nucleus is essential in fields such as nuclear physics and chemistry. The knowledge gained from studying the atomic nucleus helps us comprehend the behavior of atoms, the formation of elements, and even the functioning of nuclear reactors and atomic bombs.
Subatomic Particles
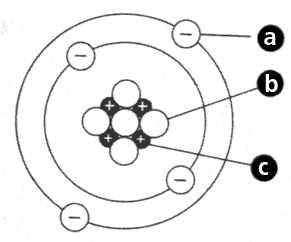
In the study of atoms, scientists have discovered several types of subatomic particles. These particles are the building blocks of atoms and play a crucial role in determining the properties and behavior of matter. The three main subatomic particles are protons, neutrons, and electrons.
Protons: Protons are positively charged particles found in the nucleus of an atom. They have a mass of 1 atomic mass unit (amu) and a charge of +1. The number of protons in an atom determines its atomic number and defines the element.
Neutrons: Neutrons are neutral particles found in the nucleus of an atom. They have a mass of 1 amu, but no charge. The number of neutrons in an atom can vary, giving rise to different isotopes of an element.
Electrons: Electrons are negatively charged particles that orbit the nucleus of an atom in energy levels or shells. They have a mass of approximately 0.0005 amu and a charge of -1. Electrons are involved in chemical reactions, bonding, and the formation of ions.
Overall, subatomic particles are essential for understanding the structure and behavior of atoms. Protons and neutrons make up the nucleus, while electrons occupy the electron cloud surrounding the nucleus. The interactions between these particles are responsible for the various chemical properties and reactions observed in matter.