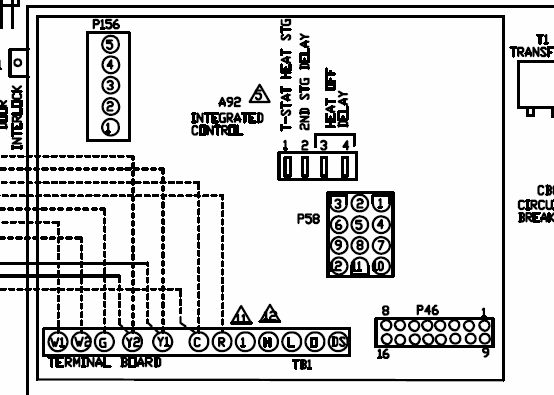
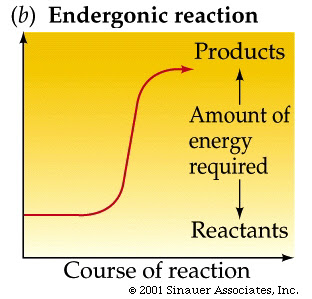
An energy diagram for an endergonic or nonspontaneous reaction is shown to the right.
The energy level of the products is higher than the energy level of the. The activation energy shown in the diagram below is for the forward reaction If the reaction were to proceed in the reverse direction (endergonic), the transition.

The Gibbs free energy graph shows whether or not a reaction is spontaneous– whether it is exergonic or endergonic. ΔG is the change in free energy.
Generally . Even a very endergonic reaction can occur if it is paired with a very exergonic one (such as hydrolysis of ATP).
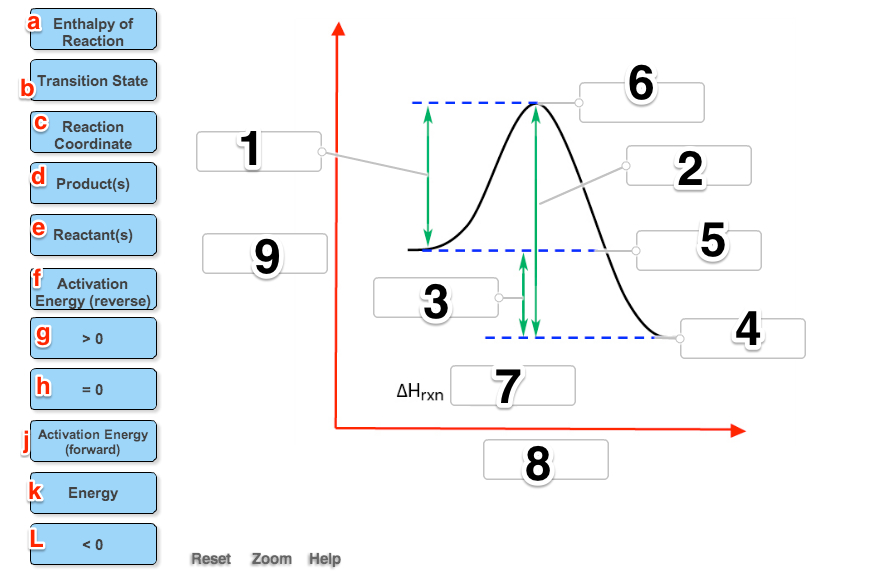
For instance, we can add up a pair of generic. Exergonic and endergonic qualifications only apply for Gibbs’ free energy. Enthalpy applies to potential energy diagrams.
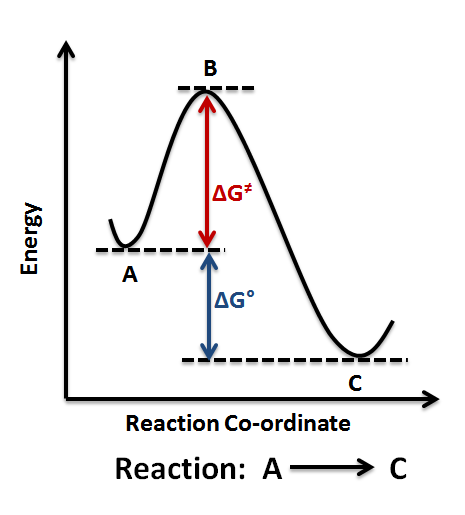
Endergonic just.Endergonic reactions and exergonic reactions are sometimes called reversible reactions. The quantity of the energy change is the same for both reactions, although the energy is absorbed by the endergonic reaction and released by the exergonic reaction. Exergonic and endergonic qualifications only apply for Gibbs’ free energy.
Enthalpy applies to potential energy diagrams. Endergonic just means that DeltaG_”rxn” > 0. So, the Gibbs’ free energy of the products is higher than the Gibbs’ free energy of the reactants.
Photosynthesis is an endergonic reaction which takes places in the natural environment. For photosynthesis, energy is supplied by sunlight.
In the human body, when endergonic reactions are taking place, most of the time the energy is supplied by the ATP. An energy hill diagram provides a visual display that shows whether a reaction is exergonic or endergonic. The diagram includes two axes, time at the bottom and the total energy of the chemical solution on the side.
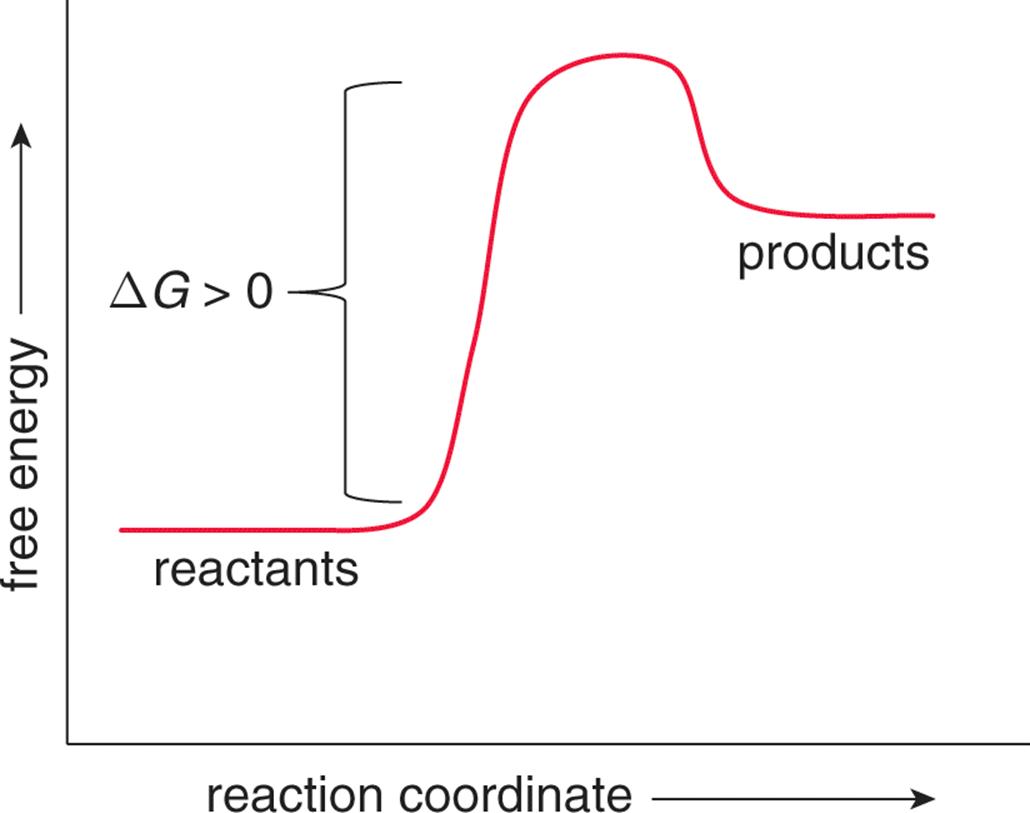
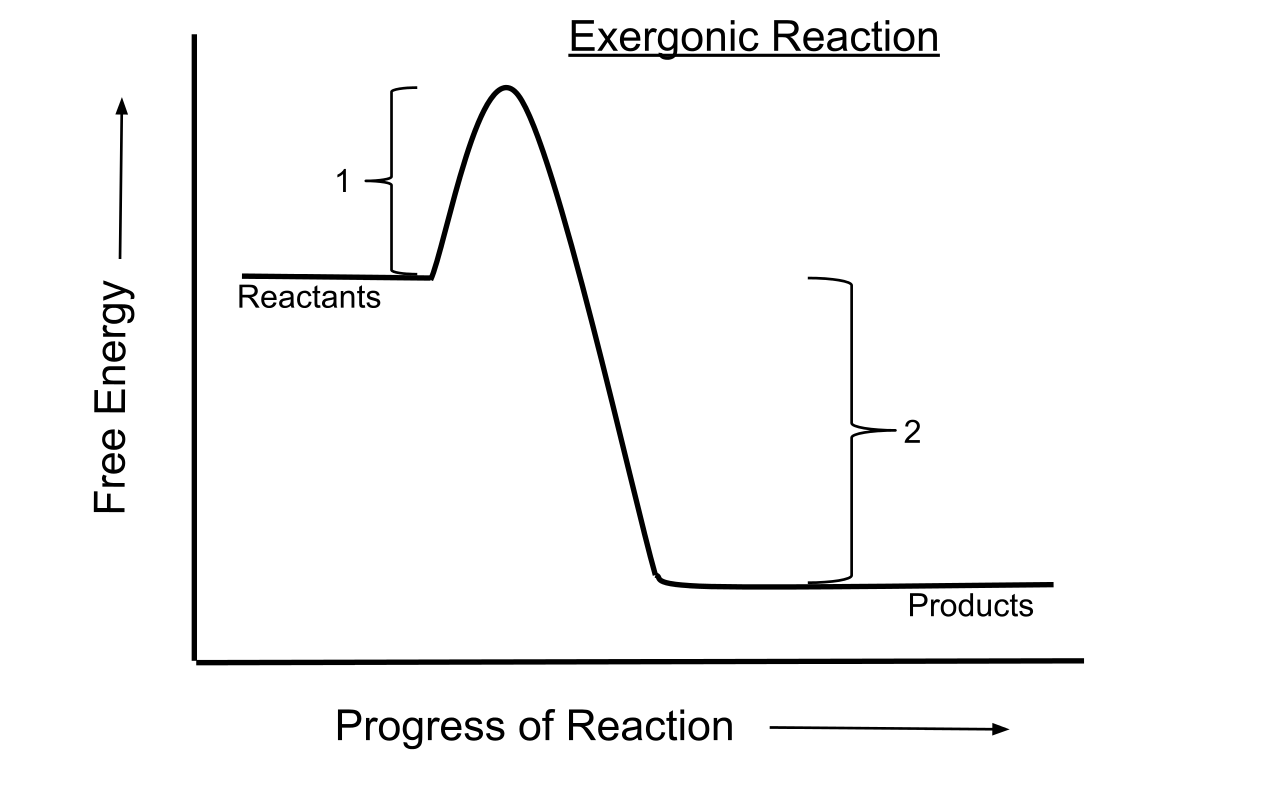
For an exergonic reaction, the amount of energy rises until the solution has enough activation energy, and then it falls. Endergonic Reaction An energy diagram for an endergonic or nonspontaneous reaction is shown to the right.

The energy level of the products is higher than the energy level of the reactants.Endergonic, exergonic, exothermic, and endothermic (video) | Khan AcademyDifference Between Endergonic and Exergonic | Definition, Explanation with Thermodynamics