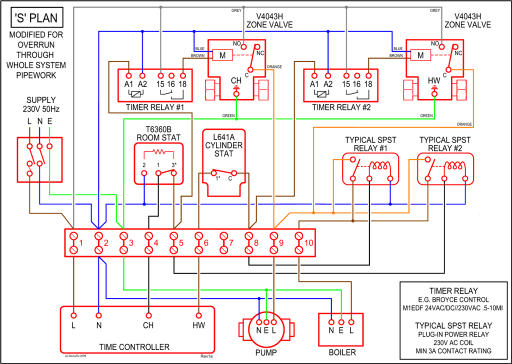
Iodine. [ATOM] [Bohr Model of Iodine], Number of Energy Levels: 5.
First Energy Level: 2. Second Energy Level: 8. Third Energy Level: Fourth Energy .
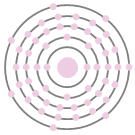
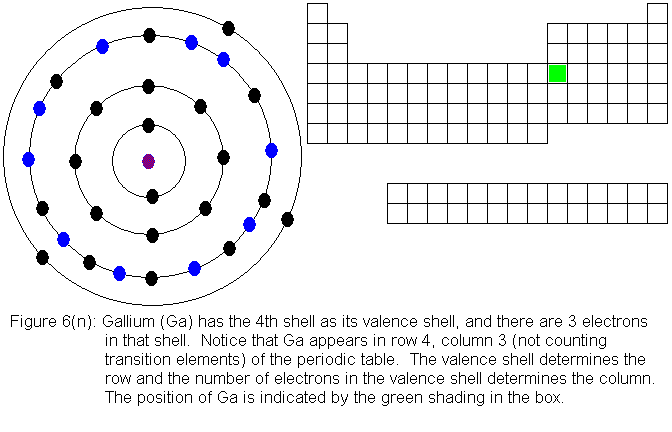
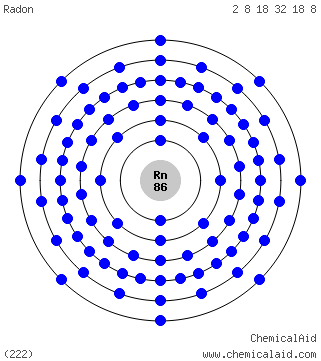
Iodine 2,8,18,18,7. I. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
In the Bohr model, electrons are. Iodine. Xe.
Xenon. Cs.
Caesium One of these models is the Bohr model, in which atoms consist of a positively. Bohr Diagram of Iodine.
Picture. How do you create a Bohr Diagram? Well, first you must know how many protons, electrons, and neutrons the element has.The Structure of Atoms Bohr-Rutherford Diagrams Bohr-Rutherford diagrams are one model that describes what an atom looks like.
Consider the atom of lithium. How to Draw Lewis Structures.
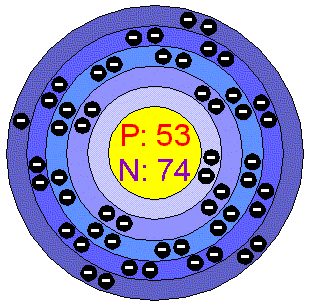
Take out a sheet of notebook paper and set up like this Bohr’s Diagram. Bohr’s diagram is . For example, in the element Iodine, the atomic number is 53, so that way we know that there are 53 protons.
Then, we could assume that the number of the protons and . In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of the Solar System, but with attraction provided by electrostatic forces rather than gravity.
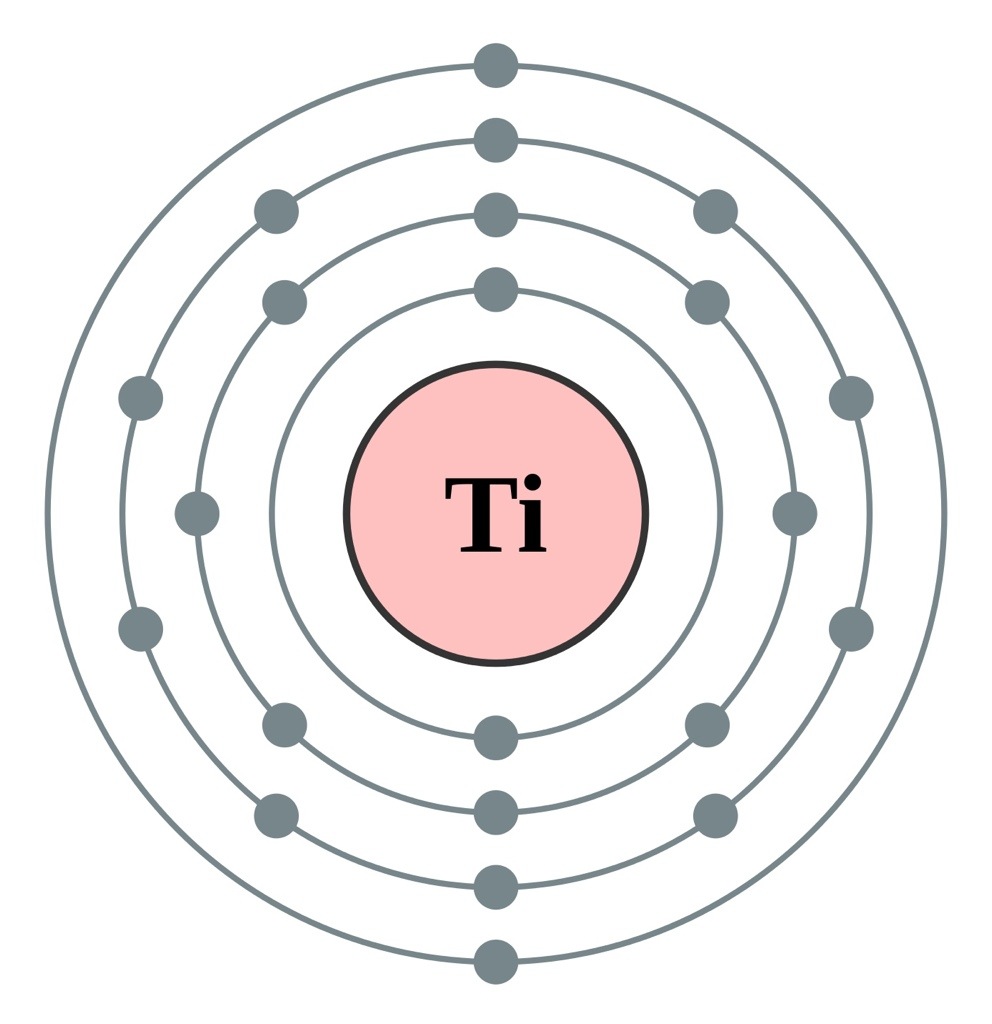
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels. Bohr Diagrams 1) Draw a nucleus with the element symbol inside.
2) Carbon is in the 2ndC period, so it has two energy levels, or shells. 3) Draw the shells around the nucleus. 8.
.File iodine (I) enhanced Bohr schematron.org – Wikimedia CommonsDiagram: Bohr Diagram Of Iodine