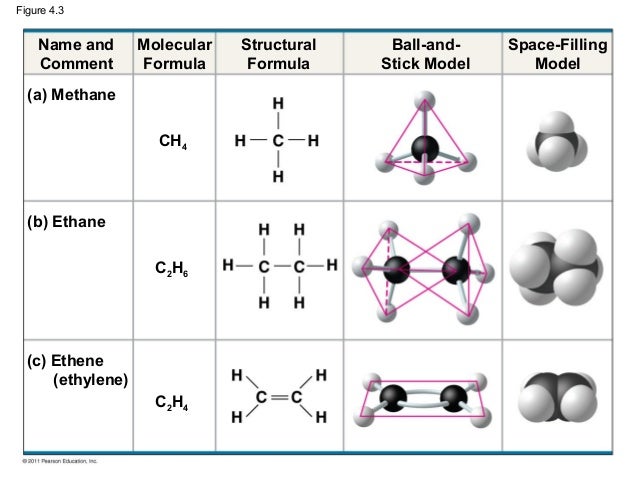
Drawing the Lewis Structure for C2H6 – Ethane. Viewing Notes: With C2H6 there are only single bonds.
Whenever you see a compound made of Carbon and. (a) Draw Lewis structures for ethane (C2H6), ethylene (C2H4), and acetylene (C 2H2). (b) What is the hybridization of the carbon atoms in each molecule?.
The first step in drawing the Lewis dot structure for ethane (C2H6) is to determine how many valence electrons are available for the molecule. After determining how many valence electrons there are in C2H6, place them around the central atom to complete the octets. The C2H6 Lewis structure has a.
H- O H • Electron Dot Diagrams show the electrons involved in the bonds of compounds.: 1 pair of C C. Electron Dot Diagrams C2H H H H C C.H All.The first step in drawing the Lewis dot structure for ethane (C_2H_6) is to determine how many valence electrons are available for the molecule.
Since C has 4 valence electrons, and each H atoms contributes 1 valence electron, the total number of electrons will be 2*4 + 6*1 = 14 “e”^(-) This means that the Lewis dot structure for C_2H_6 must account for 14 valence electrons, either through. Use information from step 4 and 5 to draw the lewis structure.
Lewis dot structure of C 2 H 6. Alternatively a dot method can be used to draw the lewis structure.
Calculate the total valence electrons in the molecule. C:4×2=8 H=1×6=6.
Total= Watch the video of Dr. B.
drawing the Lewis dot structure for C 2 H 4 (ethene) and answer the questions below.. Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons.. Hint: look at the questions before watching the video. C6H6 (Benzene): Lewis Dot Structure and Polarity Benzene is an organic compound with the molecular formula C6H6.
In this ScienceStruck post, we provide you with the polarity and steps to create the Lewis dot diagram of this aromatic compound. Drawing the Lewis Structure for C 2 H 2 (Ethyne or Acetylene) For C 2 H 2 you have a total of 10 valence electrons to work with.
In drawing the Lewis structure for C 2 H 2 (also called ethyne) you’ll find that you don’t have enough valence electrons available to satisfy the octet for .How can I write the Lewis dot structure for C2H6? | SocraticHow can I write the Lewis dot structure for C2H6?
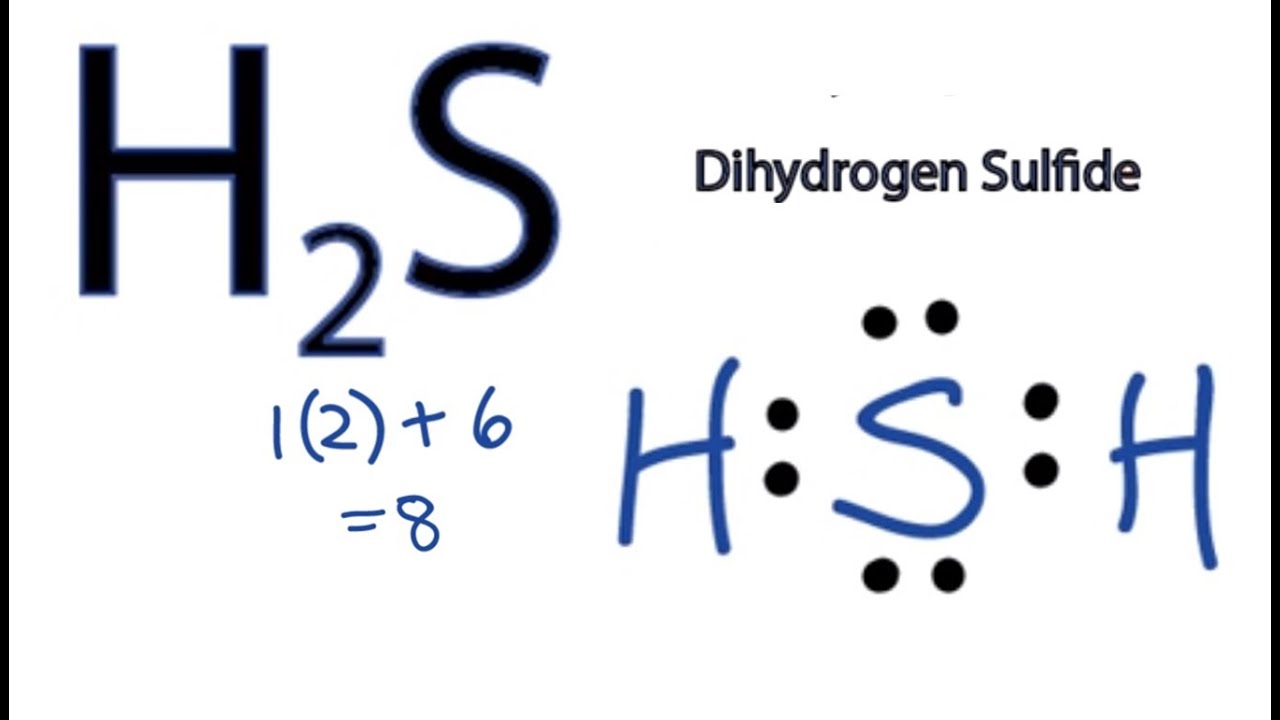
| Socratic