For the Rb+ structure use the periodic table to find the total number of valence electrons for Rb. Once we know how many valence electrons.
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
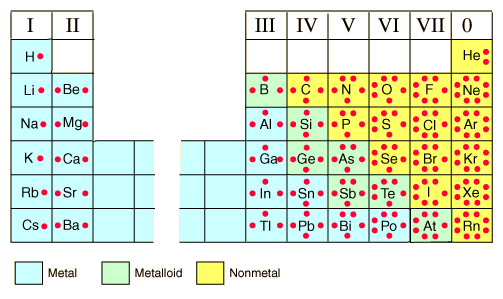
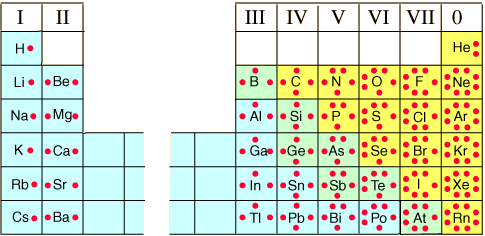
Lewis Dot Diagrams of Selected Elements. Lewis Symbols, Electron Configuration into Shells Electron Distributions Into Shells for the First Three Periods.
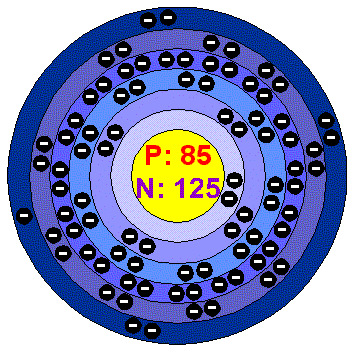
Rubidium (Rb) has an atomic mass of Find out about its Crystal Structure, Cubic: Body centered. Color, Silver Lewis Dot Diagram of Rubidium (Rb). Rubidium iodide | RbI or IRb | CID – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.Rubidium chloride is the chemical compound with the formula RbCl.
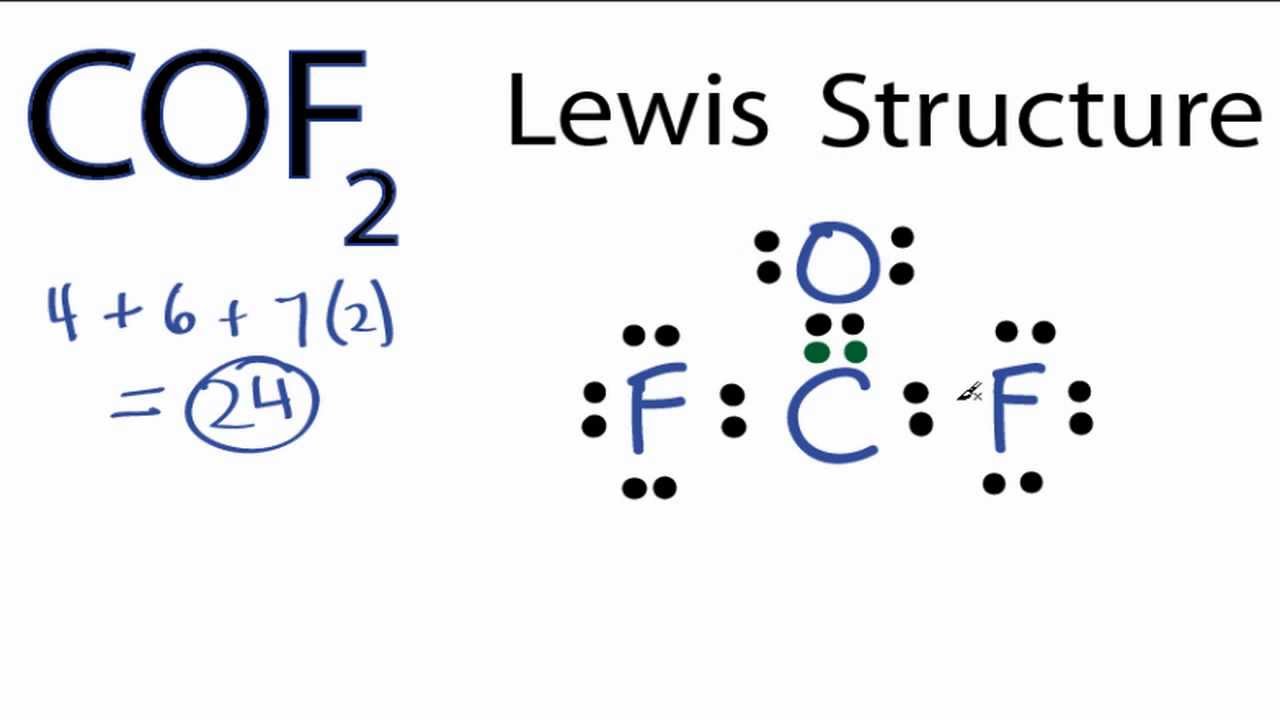
This alkali metal halide is composed of rubidium and chlorine, and finds diverse uses ranging from electrochemistry to molecular biology. The dot structure for Rubidium is Rb with a dot on the top right of b.
Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are added counterclockwise.

Anyway, a good counting place on the periodic table would be the second row (or period). It starts with hydrogen.
Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.

The Lewis structure was named after Gilbert N. Lewis, who introduced it in his article The Atom and the Molecule.
Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. The dot structure for Rubidium is Rb with a dot on the top right of b.
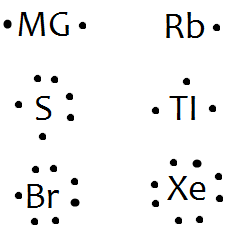
Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are adde d counterclockwise.Rubidium(1+);hydroxide | HORb2+ – PubChemWhat is the Lewis dot structure of rubidium