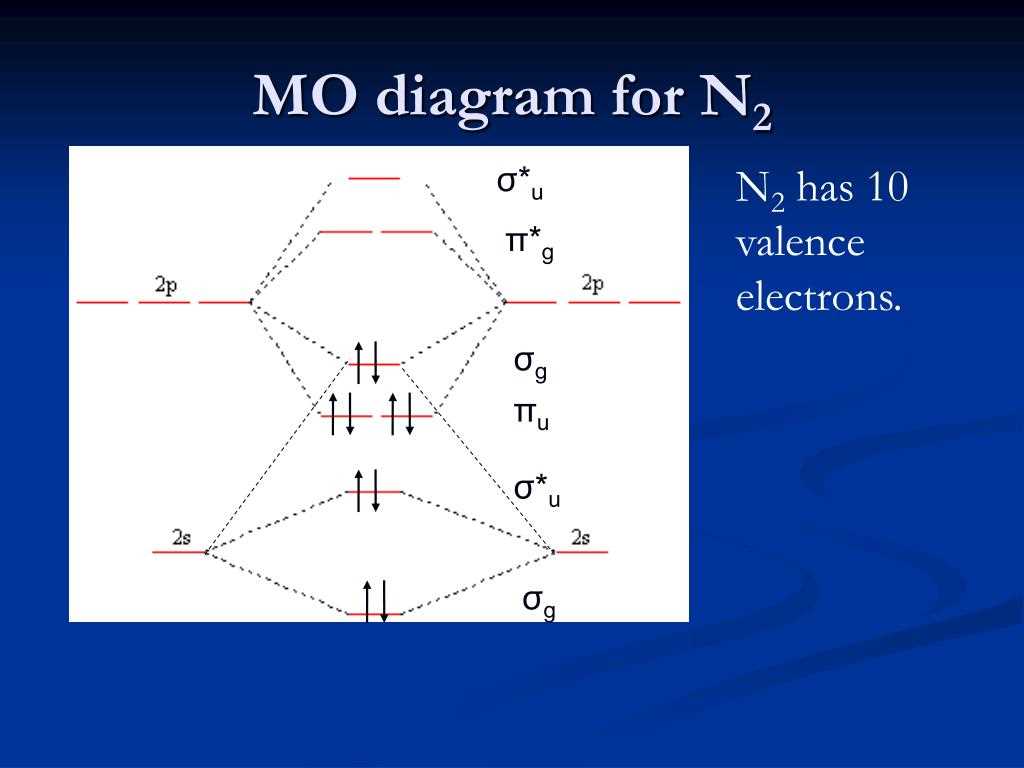
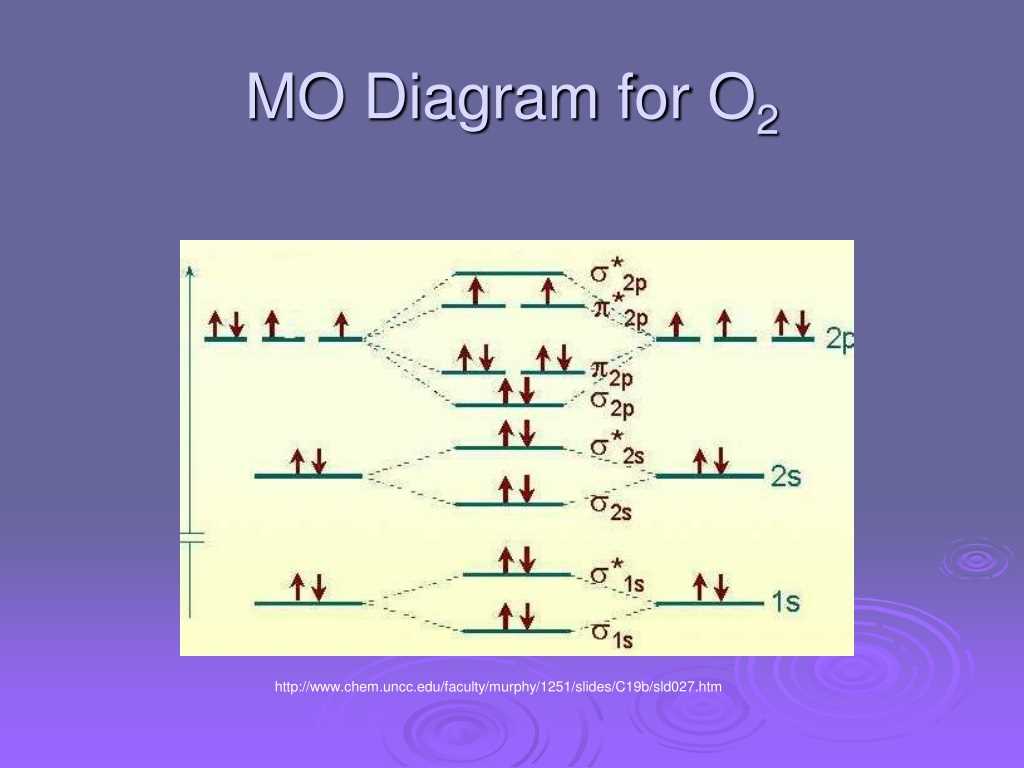
The molecular orbital (MO) diagram for O2 is a representation of the molecular orbitals formed from the overlapping of atomic orbitals in an oxygen molecule. This diagram provides valuable insight into the electronic structure and bonding properties of O2.
Oxygen is a highly reactive element with atomic number 8. In its ground state, it has 2s^2 2p^4 electron configuration, with two unpaired electrons in its p-orbitals. When two oxygen atoms combine to form a molecule, a molecular orbital is formed by the linear combination of the atomic orbitals.
The MO diagram for O2 shows two possible types of molecular orbitals: bonding (σ) and antibonding (σ*). The bonding molecular orbital is lower in energy and is formed by the constructive overlap of the atomic orbitals. This bonding orbital is responsible for the stability of the molecule. On the other hand, the antibonding molecular orbital is higher in energy and is formed by the destructive overlap of the atomic orbitals.
Mo Diagram for O2
In molecular orbital theory, the Mo diagram for O2 describes the bonding and antibonding orbitals that result from the combination of the oxygen atomic orbitals. The diagram shows the energetically favorable bonding orbitals and the energetically unfavorable antibonding orbitals.
The Mo diagram for O2 can be represented using the σ (sigma) and π (pi) notations. The σ orbitals result from the head-on overlap of the oxygen atomic orbitals, while the π orbitals are formed from the side-to-side overlap.
- The σ1s orbital is the lowest energy orbital and is fully occupied by two electrons.
- The σ*1s orbital is the antibonding orbital and is higher in energy.
- The σ2s and σ*2s orbitals are higher in energy compared to the 1s orbitals.
- The π2p orbitals result from the side-to-side overlap of the p orbitals and are at higher energy than the σ orbitals.
- The π*2p orbitals are the antibonding orbitals and have higher energy.
In the Mo diagram for O2, the two oxygen atoms contribute a total of 12 valence electrons, with each oxygen atom providing 6 electrons. The 2p orbitals of each oxygen atom overlap to form a set of π orbitals, in addition to the σ orbitals formed by the overlap of the 2s orbitals and the 2p orbitals.
The Mo diagram for O2 shows that the σ2p orbitals are filled with four electrons, while the remaining two electrons occupy one of the π2p orbitals. This results in a bond order of 2, indicating a stable double bond between the oxygen atoms. The stability of the double bond in O2 contributes to its characteristic properties, such as reactivity and the ability to support combustion.
What is a Mo diagram?
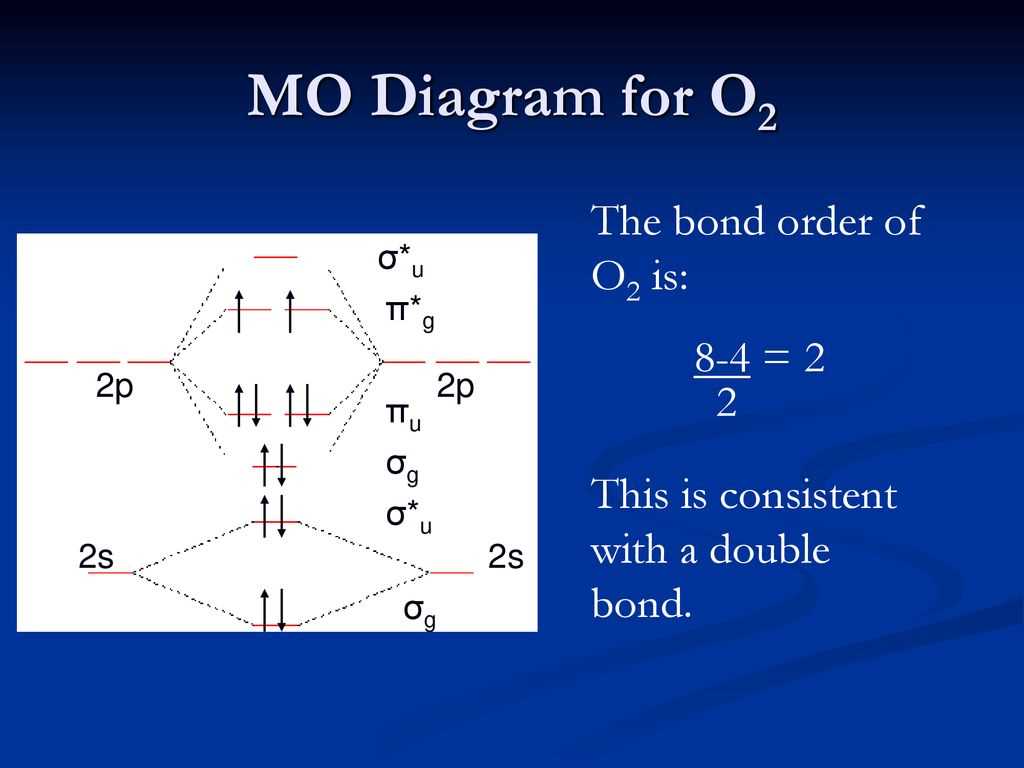
A molecular orbital (MO) diagram is a graphical representation of the molecular orbitals in a molecule. It shows the energy levels of the molecular orbitals and the distribution of electrons in these orbitals. The diagram is a useful tool for understanding the bonding and stability of a molecule.
In a MO diagram, the molecular orbitals are represented by horizontal lines, with the lower energy levels located closer to the nucleus and the higher energy levels further away. The electrons are represented by arrows, with the spin-up electrons (with their magnetic moment pointing upwards) represented by upward arrows and the spin-down electrons (with their magnetic moment pointing downwards) represented by downward arrows. The direction of the arrows indicates the spin of the electrons, which can be either “spin-up” or “spin-down”.
The MO diagram for O2, which represents the oxygen molecule, shows that the molecule has a total of 16 electrons. These electrons fill the molecular orbitals in a specific order, following the Aufbau principle and the Pauli exclusion principle. The diagram shows that there are two electrons in the sigma bonding (σ) orbital, two electrons in the sigma antibonding (σ*) orbital, four electrons in the pi bonding (π) orbitals, and eight electrons in the pi antibonding (π*) orbitals.
The MO diagram for O2 also reveals that the oxygen molecule has a double bond, as there are two electrons in the bonding orbitals and no electrons in the antibonding orbitals. This double bond contributes to the stability of the O2 molecule. The MO diagram provides a visual representation of the molecular orbitals and the electron distribution, allowing chemists to predict the properties and behavior of a molecule based on its electronic structure.
Importance of Mo diagram for O2
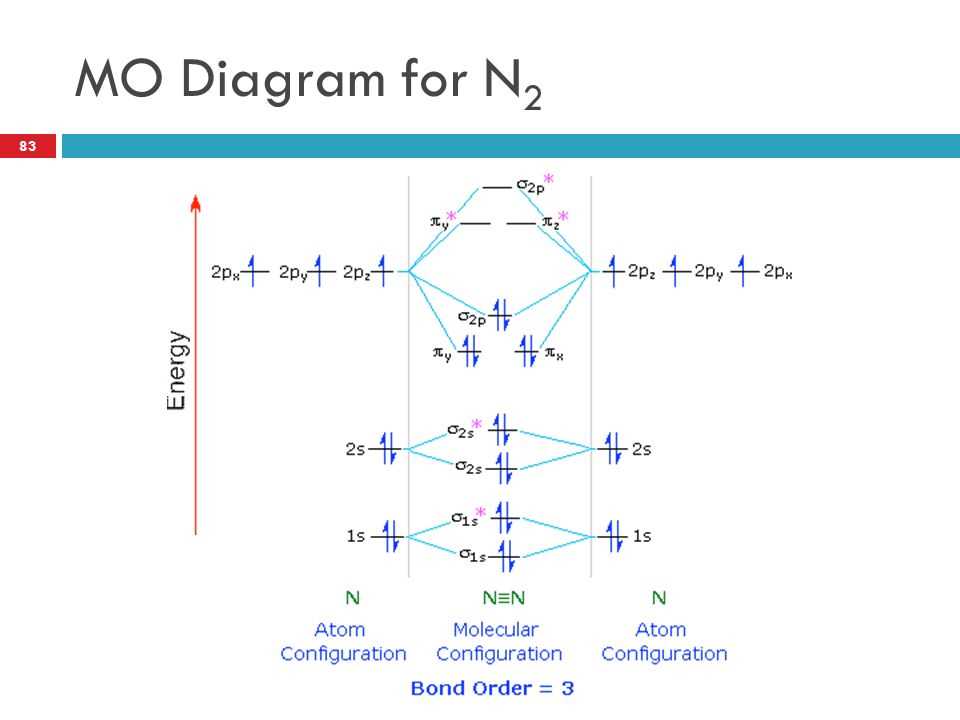
The molecular orbital (MO) diagram for O2 is of great importance in understanding the bonding and properties of oxygen molecules. The MO diagram provides information about the relative energies and arrangements of the molecular orbitals, which are formed by the combination of atomic orbitals of the oxygen atoms.
One of the key features of the MO diagram for O2 is the presence of both bonding and antibonding molecular orbitals. The bonding molecular orbitals result from the constructive interference of the atomic orbitals, while the antibonding molecular orbitals arise from the destructive interference. The distribution and occupancy of these molecular orbitals determine the stability and reactivity of O2.
In the MO diagram for O2, the bonding molecular orbitals are lower in energy compared to the atomic orbitals, indicating the formation of a stable molecule. The highest occupied molecular orbital (HOMO) is responsible for the covalent bonding between the oxygen atoms, while the lowest unoccupied molecular orbital (LUMO) is involved in the reactivity of O2, such as its ability to accept electrons.
By analyzing the MO diagram for O2, scientists can predict the bond strength, bond length, and other properties of the oxygen molecule. It also helps in understanding the concept of bond order, which is derived from the difference between the number of electrons in bonding and antibonding orbitals. The bond order indicates the strength of the bond and is directly related to the stability of the molecule.
The MO diagram for O2 also provides insights into the electronic structure and behavior of other oxygen-containing compounds and ions. It serves as a foundation for studying the reactivity and properties of these compounds, which are important in various fields such as chemistry, biology, and materials science.
In conclusion
Overall, the MO diagram for O2 is essential for understanding the bonding, stability, and reactivity of oxygen molecules and related compounds. It offers valuable insights into the electronic structure and properties of these substances, playing a crucial role in the field of chemistry.
Key components of a Mo diagram for O2
In order to understand the molecular orbitals (MOs) of oxygen (O2), it is essential to consider certain key components of its MO diagram. These components include the electron configuration of oxygen, the combination of atomic orbitals, and the resulting MO energies and electron occupancy.
Oxygen has a total of 16 electrons, with each oxygen atom contributing 8 electrons. The electron configuration of oxygen is 1s^2 2s^2 2p^4, where 1s, 2s, and 2p are the atomic orbitals. To construct the MO diagram, these atomic orbitals combine to form molecular orbitals through the process of orbital overlap. The 2s orbital and two of the 2p orbitals (2px and 2py) overlap in-phase to form σ2s, σ2px, and σ2py bonding molecular orbitals, while the remaining 2p orbital (2pz) remains non-bonding.
The resulting MO energies depend on the energy level splitting caused by the interaction of the atomic orbitals. The σ2s bonding molecular orbital is lower in energy than the non-bonding 2pz orbital, while the σ2px and σ2py bonding molecular orbitals are higher in energy. Additionally, antibonding molecular orbitals are formed by out-of-phase overlap, where the antibonding σ*2s orbital is higher in energy than the 2pz orbital, and the antibonding σ*2px and σ*2py orbitals are higher in energy than the corresponding bonding orbitals.
The electron occupancy of the MOs is determined by the Aufbau principle and Hund’s rule. The 2s orbital is fully occupied with two electrons, while the remaining 2pz, σ2px, and σ2py orbitals each have one electron. The total number of bonding electrons in the MO diagram is equal to the number of electrons in the valence shells of the oxygen atoms, which is 8. The presence of unpaired electrons in the bonding orbitals contributes to the paramagnetic nature of O2.
Understanding the bonding in O2 using a Mo diagram
The bonding in O2 can be understood using a molecular orbital (MO) diagram. In this diagram, the valence electrons are represented by the atomic orbitals of the oxygen atoms that combine to form molecular orbitals. The MO diagram helps us visualize the distribution of electrons in the molecular orbitals and determine the stability and reactivity of the molecule.
In the MO diagram for O2, the two oxygen atoms each contribute six valence electrons, resulting in a total of 12 valence electrons. These electrons occupy a series of molecular orbitals, which are formed by the overlapping of the atomic orbitals. The lowest energy molecular orbital is called the bonding orbital (σ2s), which is formed by the constructive overlap of the two 2s orbitals of the oxygen atoms. This bonding orbital is filled with two electrons, one from each oxygen atom.
In addition to the bonding orbital, there are two antibonding orbitals (σ*2s and σ*2p) that are formed by the destructive overlap of the 2s and 2p orbitals, respectively. These antibonding orbitals are higher in energy than the bonding orbital and are empty in the ground state of O2. The remaining six valence electrons occupy the two remaining molecular orbitals, which are the σ2p bonding orbital and the σ*2p antibonding orbital. This distribution of electrons results in a net bond order of 2, indicating a stable and covalently bonded O2 molecule.
In summary, the MO diagram for O2 shows the distribution of electrons in the molecular orbitals, indicating the bonding and antibonding interactions between the oxygen atoms. The filled bonding molecular orbitals contribute to the stability of the molecule, while the empty antibonding molecular orbitals do not contribute to the overall bonding. Understanding the MO diagram helps us understand the nature of the chemical bond in O2 and other molecules.
Applications of Mo diagram for O2
Mo diagram for O2 has several applications in the field of chemistry. Here are some of the key applications:
- Bonding analysis: The Mo diagram for O2 provides insights into the nature and strength of the chemical bonding in the oxygen molecule. It helps in understanding the formation of the O2 bond and the distribution of electron density between the bonding and antibonding orbitals.
- Reaction prediction: The Mo diagram can be used to predict the reactivity of oxygen and its involvement in various chemical reactions. It helps in understanding the likelihood of O2 participating in oxidation-reduction reactions, as well as its ability to act as an electrophile or nucleophile in different reaction conditions.
- Electronic structure analysis: The Mo diagram provides information about the electronic structure of the oxygen molecule, including the energy levels and electron configuration of the bonding and antibonding orbitals. This information is useful for studying the spectroscopic properties and electronic transitions of O2.
- Bond length and strength: The Mo diagram can be used to estimate the bond length and bond strength of the O2 molecule. By analyzing the relative energies of the bonding and antibonding orbitals, one can predict the stability of the O2 bond and its susceptibility to breaking or forming new bonds.
In conclusion, the Mo diagram for O2 is a valuable tool for understanding the bonding, reactivity, electronic structure, and physical properties of the oxygen molecule. It has widespread applications in various branches of chemistry, including organic chemistry, inorganic chemistry, and physical chemistry.