In the field of chemistry, molecular orbital diagram is a powerful tool used to visualize and understand the electronic structure of molecules. It is based on the principles of quantum mechanics, which describe the behavior of electrons in atoms and molecules. By representing the distribution of electrons in different molecular orbitals, these diagrams provide valuable insights into a molecule’s stability, reactivity, and physical properties.
One example of a molecular orbital diagram is that of a diatomic molecule, such as hydrogen (H2). In this case, the diagram shows two energy levels, each corresponding to a different molecular orbital. The lower energy level is called the bonding molecular orbital, or the sigma orbital, and it is formed by the constructive interference of the atomic orbitals of the two hydrogen atoms. The higher energy level is called the anti-bonding molecular orbital, or the sigma star orbital, and it is formed by the destructive interference of the atomic orbitals.
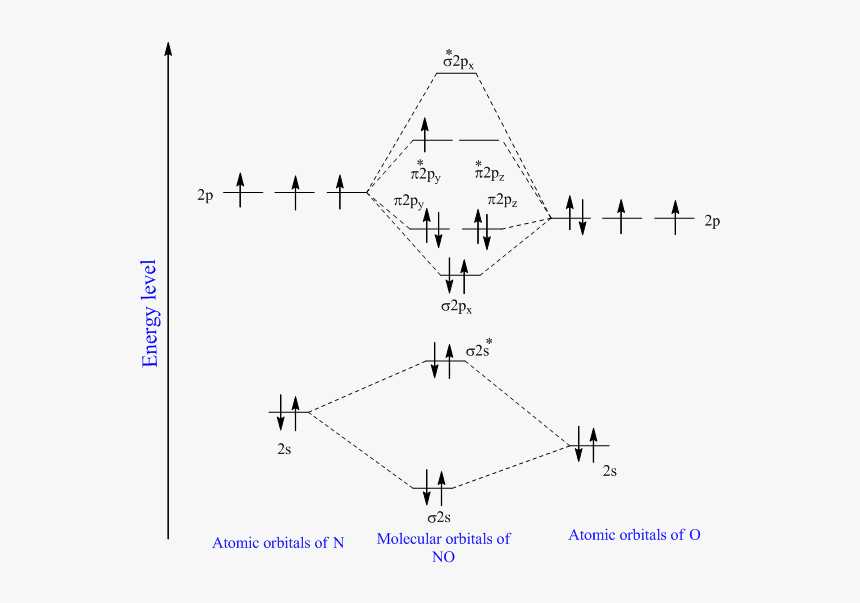
Another example is the molecular orbital diagram of nitrogen (N2). In this case, there are three energy levels: the bonding sigma orbital, the anti-bonding sigma star orbital, and the non-bonding pi orbital. The bonding sigma orbital is lower in energy than the anti-bonding sigma star orbital, which indicates that the molecule is stable. The non-bonding pi orbital is higher in energy and does not contribute to bonding or anti-bonding interactions.
Example 1: Hydrogen molecule (H2)
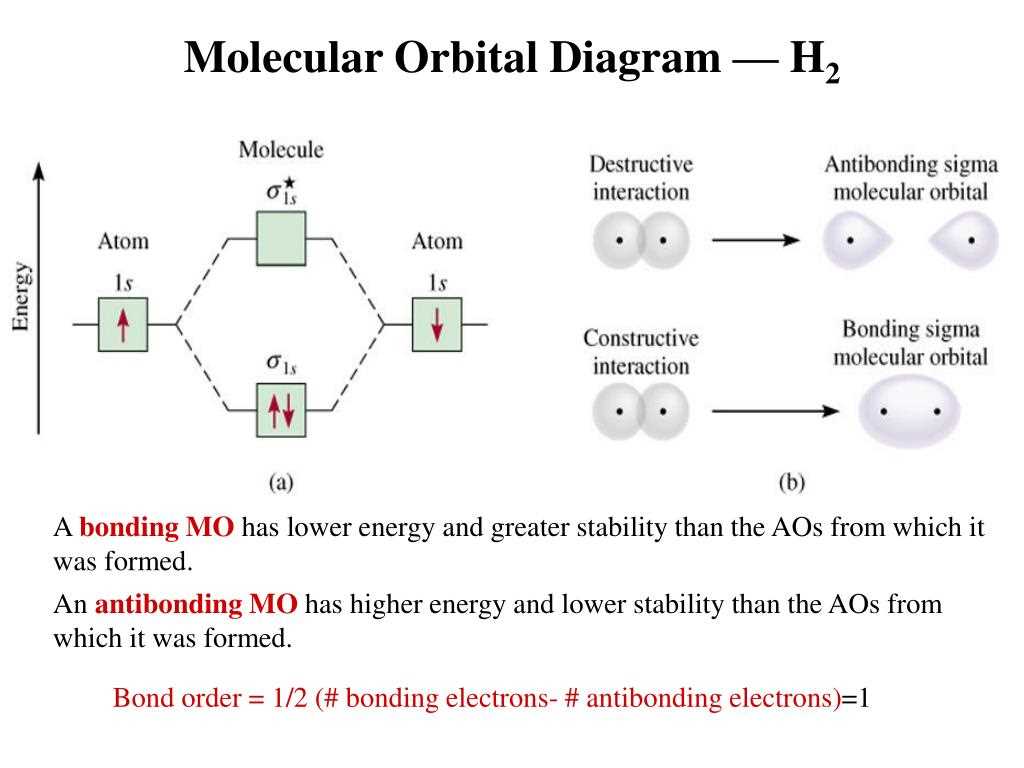
The molecular orbital diagram for the hydrogen molecule (H2) involves the combination of two hydrogen atomic orbitals to form molecular orbitals. Each hydrogen atom has one electron in its 1s orbital, which combines to form two molecular orbitals: a bonding orbital and an antibonding orbital.
The bonding molecular orbital, also known as the sigma (σ) bonding orbital, is formed by the combination of the two 1s atomic orbitals. This orbital is lower in energy than the original atomic orbitals and can hold two electrons with opposite spins, resulting in a stable covalent bond between the two hydrogen atoms.
The antibonding molecular orbital, known as the sigma-star (σ*) antibonding orbital, is formed by the combination of the two 1s atomic orbitals with a phase difference of 180 degrees. This orbital is higher in energy than the original atomic orbitals and is empty in the ground state of the molecule. It does not contribute to the stability of the molecule.
The molecular orbital diagram for H2 can be represented as follows:
| Molecular Orbital | Energy | Electron Configuration |
|---|---|---|
| Bonding (σ) | Lower energy | ↑↓ |
| Antibonding (σ*) | Higher energy | Empty |
This molecular orbital diagram illustrates that the two electrons in the hydrogen molecule occupy the bonding orbital, resulting in a stable H2 molecule. The bond formed between the two hydrogen atoms is a covalent bond, where the electrons are shared between the atoms in the bonding orbital.
Example 2: Oxygen molecule (O2)
The oxygen molecule (O2) is a diatomic molecule consisting of two oxygen atoms. Like other diatomic molecules, the oxygen molecule can be represented using a molecular orbital diagram, which illustrates the formation of molecular orbitals through the overlap of atomic orbitals.
The oxygen molecule contains a total of 16 valence electrons, with each oxygen atom contributing 8 electrons. Using Hund’s rule and the Aufbau principle, we can construct a molecular orbital diagram for O2.
The molecular orbital diagram for O2 consists of a sigma bonding orbital (σ2p) and a sigma antibonding orbital (σ*2p). The σ2p orbital is formed by the overlap of two 2p atomic orbitals, while the σ*2p orbital is formed by the antibonding combination of the two 2p atomic orbitals.
In the σ2p bonding orbital, there are 4 electrons (2 from each oxygen atom) that occupy the lower energy level. This bonding orbital stabilizes the molecule, contributing to the overall strength of the O2 bond. In contrast, the σ*2p antibonding orbital has 4 electrons at higher energy levels, which weakens the O2 bond.
In summary, the molecular orbital diagram for O2 shows the formation of a σ2p bonding orbital and a σ*2p antibonding orbital. The bonding orbital stabilizes the molecule, while the antibonding orbital weakens the bond. This molecular orbital diagram helps to explain the properties and behavior of the oxygen molecule.
Example 3: Ethene molecule (C2H4)
Ethene, also known as ethylene, is a hydrocarbon with the chemical formula C2H4. It consists of two carbon atoms bonded together by a double bond and four hydrogen atoms. Ethene is an important industrial chemical used for the production of plastics, solvents, and various other chemicals.
The molecular orbital diagram of ethene can be constructed by combining the atomic orbitals of carbon and hydrogen atoms. The two carbon atoms each contribute one 2p orbital, which hybridizes to form two sp2 hybrid orbitals and one unhybridized p orbital. The sp2 hybrid orbitals overlap with each other, forming a sigma bond, while the unhybridized p orbitals overlap sideways, forming a pi bond. The sigma bond is stronger and more stable than the pi bond.
In the molecular orbital diagram of ethene, the two carbon 1s orbitals are the lowest-energy orbitals, followed by the two carbon 2s orbitals. The two carbon sp2 hybrid orbitals and the hydrogen 1s orbitals are at slightly higher energy levels. Finally, the unhybridized carbon p orbital and the hydrogen 1s orbitals are the highest-energy orbitals.
The bonding molecular orbitals of ethene are formed by the constructive interference of the atomic orbitals, while the antibonding molecular orbitals are formed by the destructive interference. The lowest-energy bonding molecular orbital is the sigma bonding orbital, followed by two pi bonding orbitals. The highest-energy antibonding orbitals are the pi* antibonding orbitals, followed by the sigma* antibonding orbital.
In summary, the molecular orbital diagram of ethene shows that the carbon-carbon double bond consists of one sigma bond and one pi bond. The sigma bond is formed by the overlap of the sp2 hybrid orbitals, while the pi bond is formed by the overlap of the unhybridized p orbitals. This molecular orbital diagram provides insights into the bonding and stability of the ethene molecule.
Example 4: Benzene molecule (C6H6)
Benzene (C6H6) is a cyclic aromatic hydrocarbon consisting of a ring of six carbon atoms with alternating single and double bonds. The molecular formula can be represented as (C6H6) or as a hexagon, where each corner represents a carbon atom and each line represents a bond.
To construct the molecular orbital diagram for benzene, we start by considering the atomic orbitals of the carbon atoms. Each carbon atom in benzene has two 2p orbitals perpendicular to the plane of the molecule. These 2p orbitals will combine to form six molecular orbitals: three bonding and three antibonding.
The molecular orbital diagram of benzene can be represented as follows:
| Molecular Orbital | Energy Level | Number of Electrons |
|---|---|---|
| π Molecular Orbital (bonding) | Lower | 6 |
| σ Molecular Orbital (bonding) | Intermediate | 0 |
| π Molecular Orbital (nonbonding) | Intermediate | 0 |
| π Molecular Orbital (antibonding) | Intermediate | 0 |
| σ Molecular Orbital (antibonding) | Higher | 0 |
| π Molecular Orbital (antibonding) | Higher | 0 |
In benzene, all six electrons from the six carbon atoms occupy the lower energy level π molecular orbital, resulting in a delocalized π electron system. This delocalized system of electrons is responsible for the stability and aromaticity of benzene.
In summary, the molecular orbital diagram of benzene consists of six molecular orbitals: three bonding, one nonbonding, and two antibonding orbitals. The delocalized π electron system in benzene contributes to its stability and aromatic nature.