This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be .
Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure “Molecular orbital.
This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
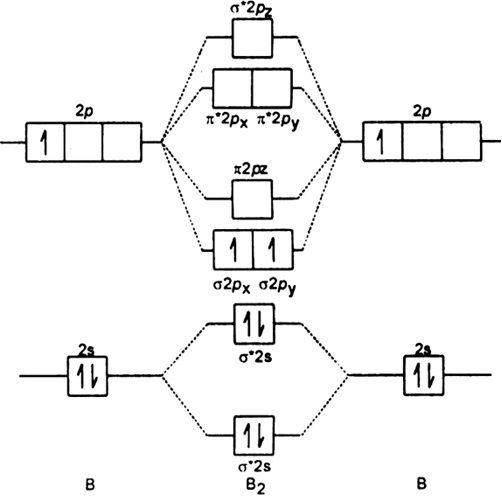
Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature.
6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.

The same method can be applied to for other diatomic molecules, but involving more than the 1 s atomic orbitals. Describe the essential difference between a sigma and a pi molecular orbital. Define bond order, and state its significance.
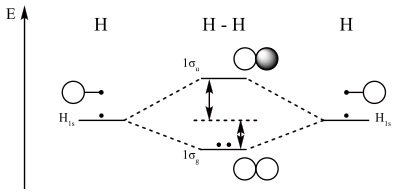
Construct a “molecular orbital diagram” of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. A molecule in which all the electrons are paired, is called diamagnetic while molecule which has one or more unpaired electron is called paramagnetic. Molecular orbital diagram of H 2 (Hydrogen molecule). Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.
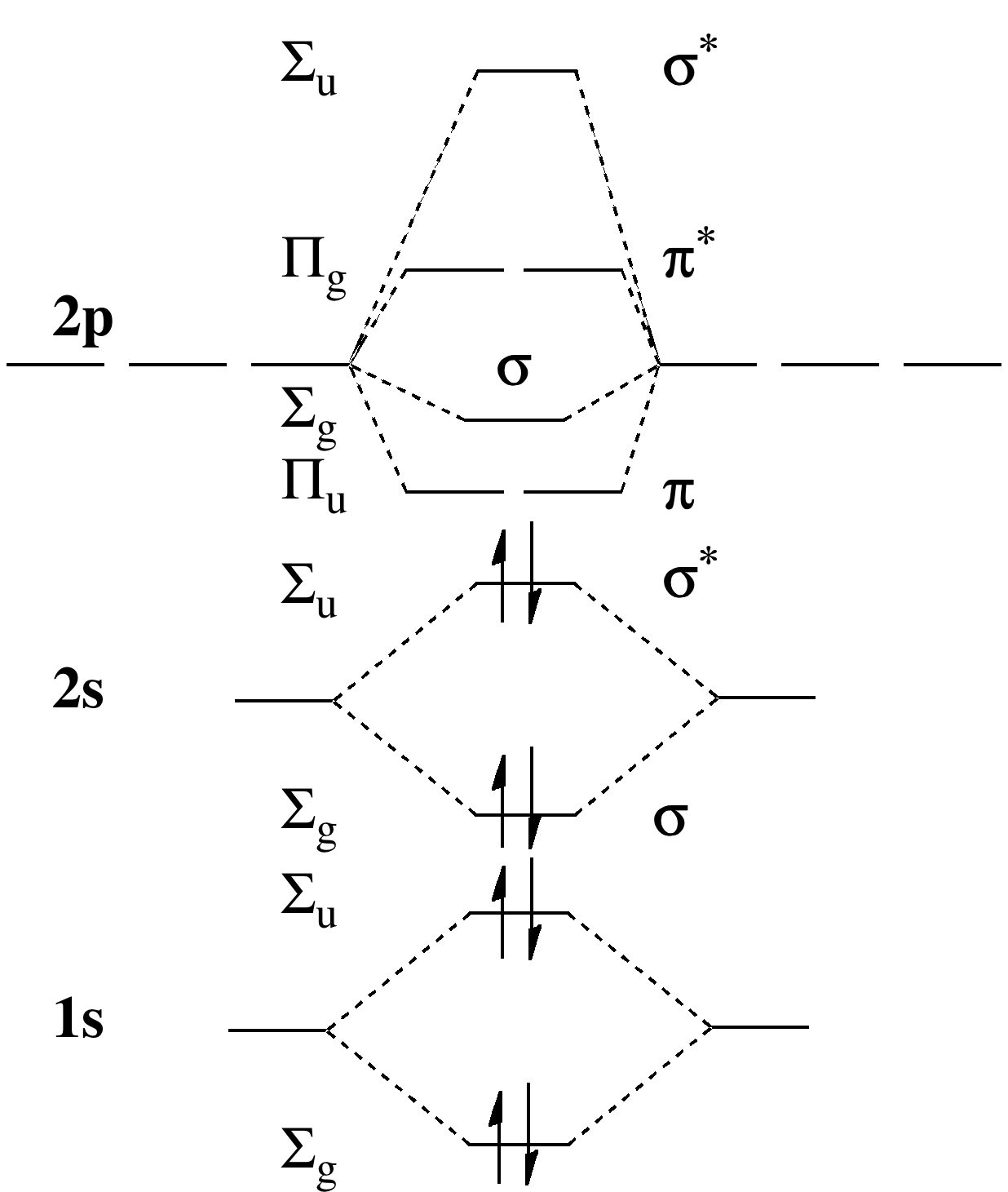
Molecular orbital (MO) diagram for N2 and N2^- $-$\mathrm{p}$ interaction moving from $\ce{Li2}$ to $\ce{F2}$. The $\mathrm{s}$-$\mathrm{p}$ interaction is the bonding interaction between the $\mathrm{2s}$ orbital of one atom and the $\mathrm{2p_{z}}$ orbital of another atom which (among other things) increases the energy of the $\mathrm.Molecular orbitals of diatomic molecules.Molecular orbital diagram – Wikipedia
