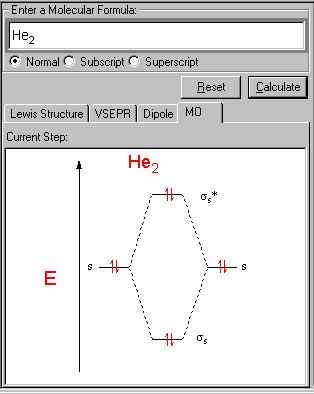
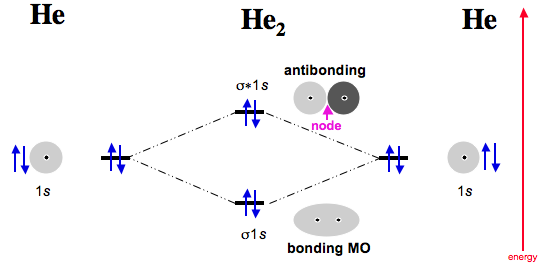
He2 is not possible. He MO Diagram. Eg: He + H; same mixing as above.
Three electrons, two in sigma, one in sigma*. One more electron in.
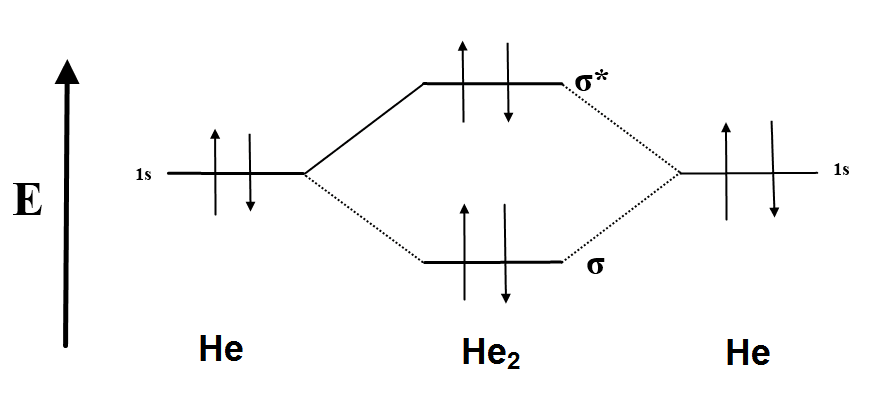
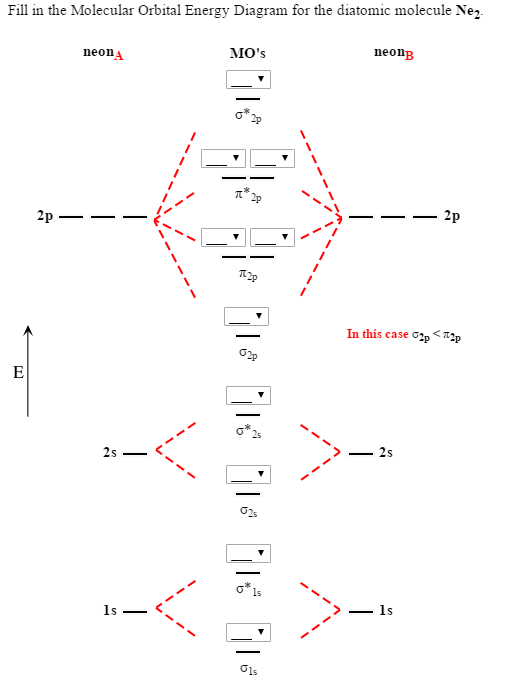
In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4.
Molecular. Chemical bonding – Molecular orbitals of H2 and He2: The procedure can be The molecular orbital energy-level diagram, which is a diagram that shows the.
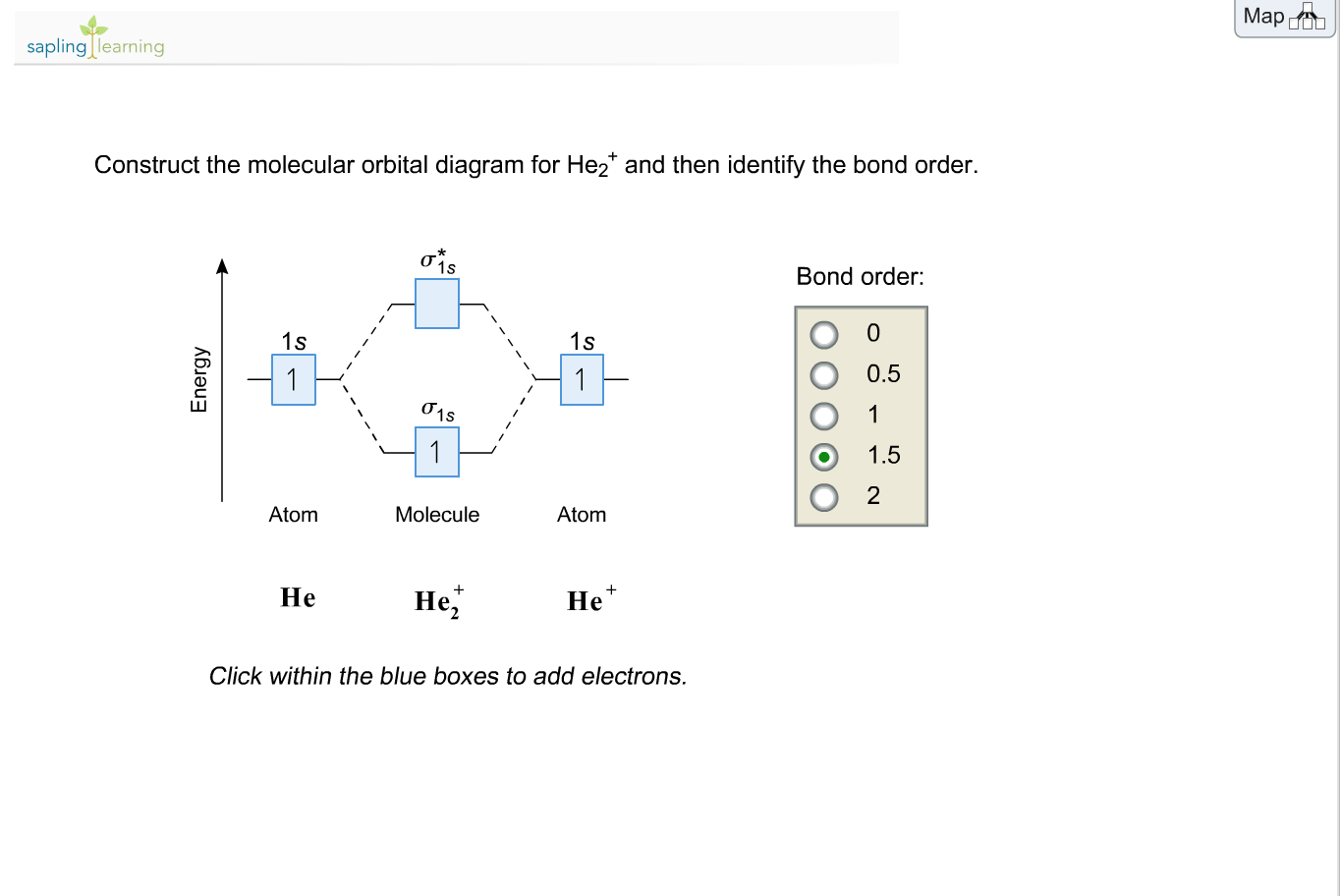
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe.
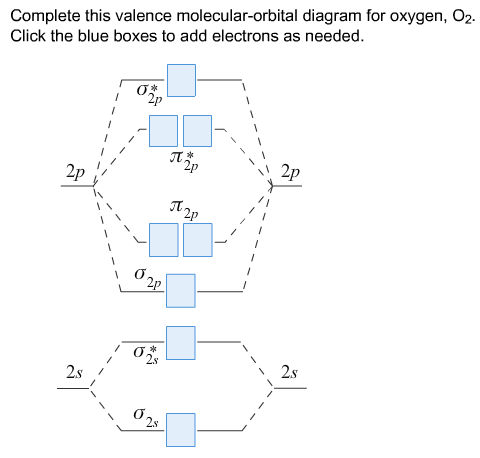
According to the molecular orbital theory, in a supposed He2 molecule, both the bonding and the antibonding orbitals will have 2 electrons each. And so, the.Show transcribed image text Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons.
Best answer. % (1 rating) Get this answer with Chegg Study View this answer.
Previous question Next question%(16). Please note the diagram is for He2+ but the He-H is very similar Eg: Li + H; Li has 1s + 2s, while H has 1s.
This mix to form a sigma orbital from H1s+Li2s, a sigma* orbital and H1s-Li2s, and a non bonding orbital from Li1s (lower in energy than the sigma). After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide molecules and some of their positive ions for the range of internuclear distances from about times equilibrium down to bohr. The orbital correlation diagram in predicts the same thing–two electrons fill a single bonding molecular orbital.
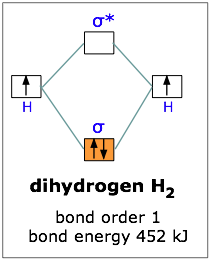
To further demonstrate the consistency of the Lewis structures with M.O. theory, we will formalize a definition of bond order–the number of bonds between atoms in a molecule.
The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at lowest energy and the orbital with nodal planes between all the atoms lying at highest energy.Diatomic Species | MO theory | ChemogenesisMolecular Orbital Theory