Three basic events must take place for the reaction to occur; The atoms, molecules, The activation energy can be determined by plotting the natural log of the rate constant vs.
the inverse temperature. 21 .
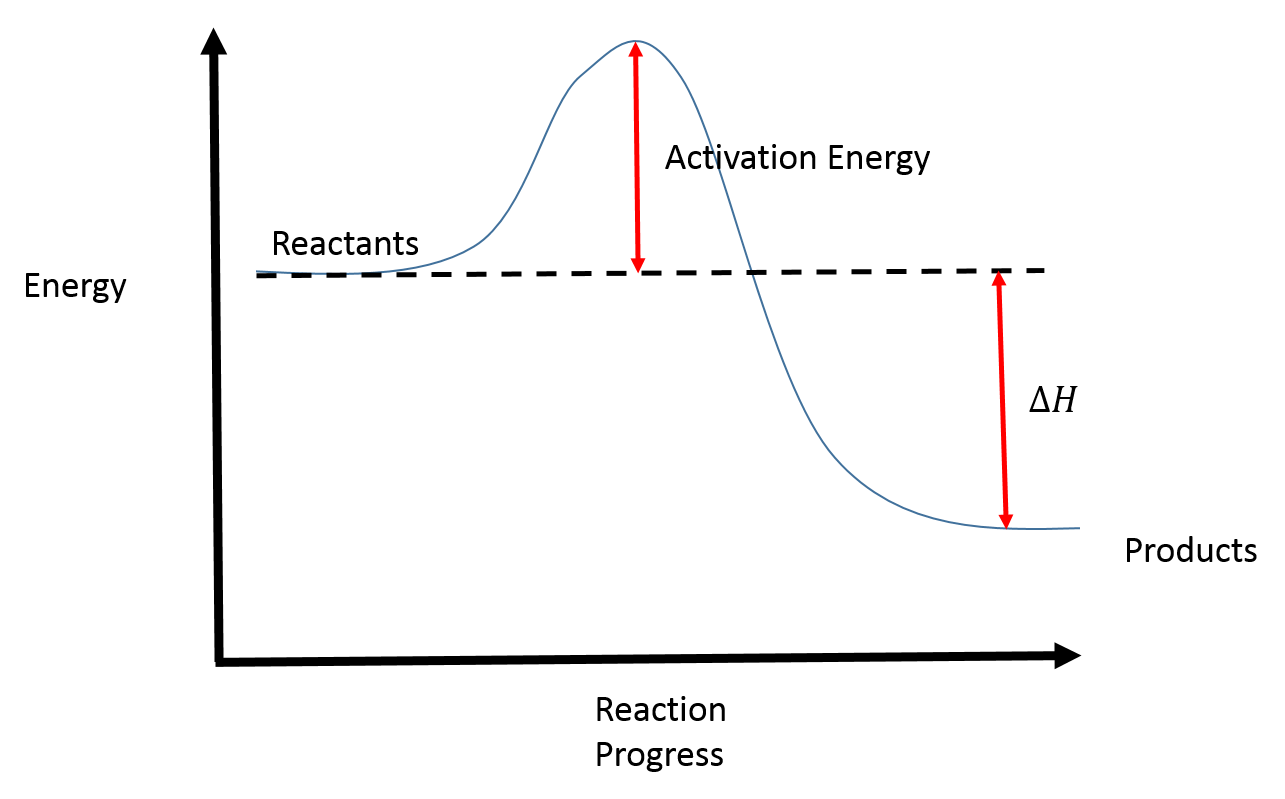
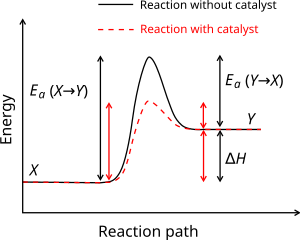
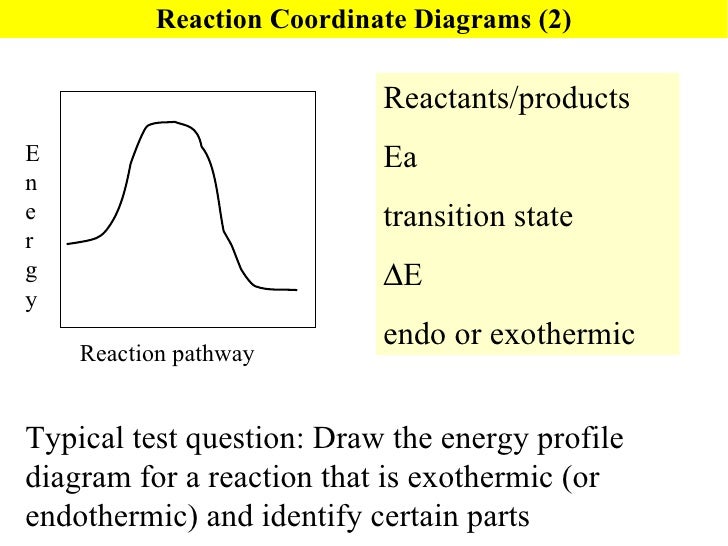
Is it exothermic or endothermic?. A reaction will be exothermic if the energy of the products is less than the energy of the Below is a reaction coordinate diagram for an endothermic reaction.
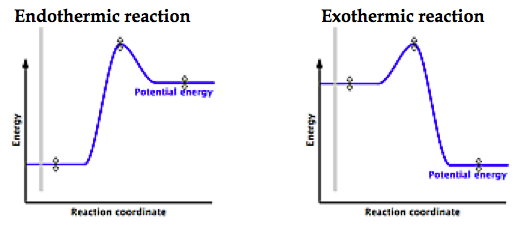
A general Reaction Coordinate Diagram relating the energy of a system to leading to an exothermic reaction (∆H 0). Endothermic Reaction.
One of your salts generated an endothermic reaction with water, while the other salt generated an . Energy diagrams for endothermic and exothermic reactions.
This is shown in an Energy vs Reaction Coordinate plot. useful in showing: 1) whether a reaction is an exo- or endothermic process, 2) which reaction Exothermic reactions will have products that are lower in energy than the reactants.Endothermic Reaction Energy Diagram – 62 Unique Figure Of Endothermic Reaction Energy Diagram, thermochemistry Exothermic and Endothermic Reactions,bbc Gcse Bitesize Science Energy From Reactions,eilisha Joy Bryson,why is An Exothermic Reaction Spontaneous Example,igcse Chemistry 4 14 Represent Exothermic and Endothermic revision questions.
Endothermic vs. exothermic reactions.
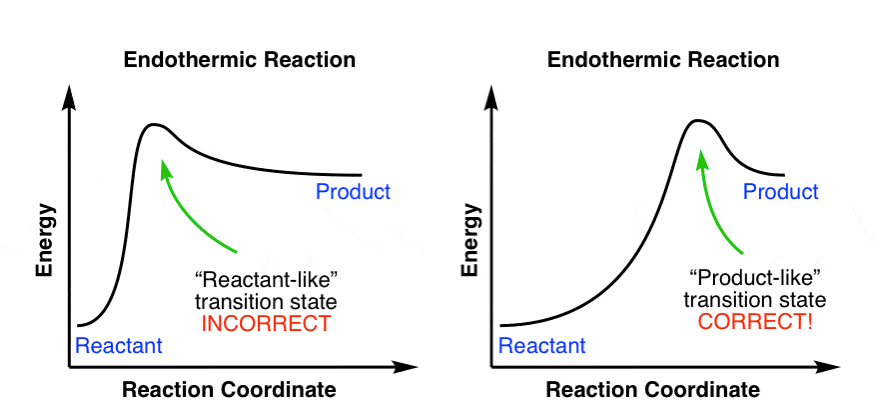
“When trying to classify a reaction as exothermic or endothermic, watch how the temperature of the surrounding—in this case, the flask—changes. the reactants are at a lower energy level compared to the products—as shown in the energy diagram below.
In other words, the products are less. A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction!
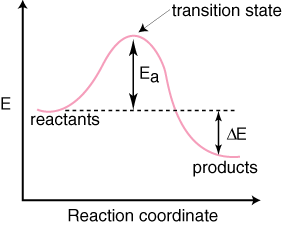
2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic.
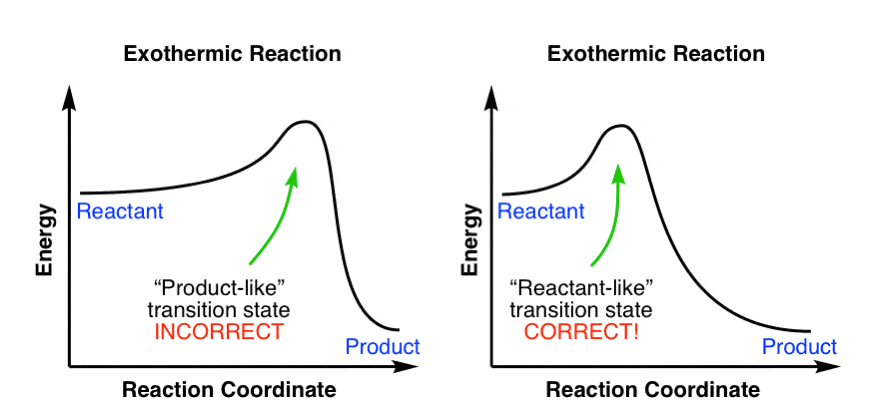
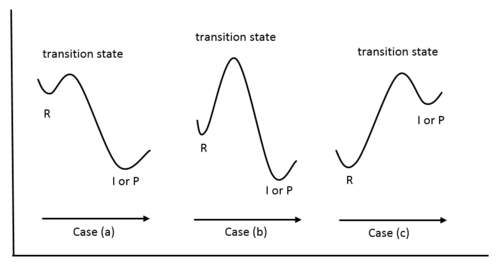
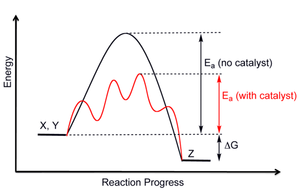
Figure 8: Reaction Coordinate Diagrams showing favorable or unfavorable and slow or fast reactions [7] The relative stability of reactant and product does not define the feasibility of any reaction all by itself. A reaction with a negative ∆H° is called an exothermic reaction.
If the bonds that are formed are weaker than those that are broken, ∆H° will be positive. A reaction with a positive ∆H° is called an endothermic reaction.
Entropy (∆S°) is defined as the degree of disorder. It is a .Analyzing Energy With a Reaction Coordinate Diagram | schematron.orgEnergy profile (chemistry) – Wikipedia