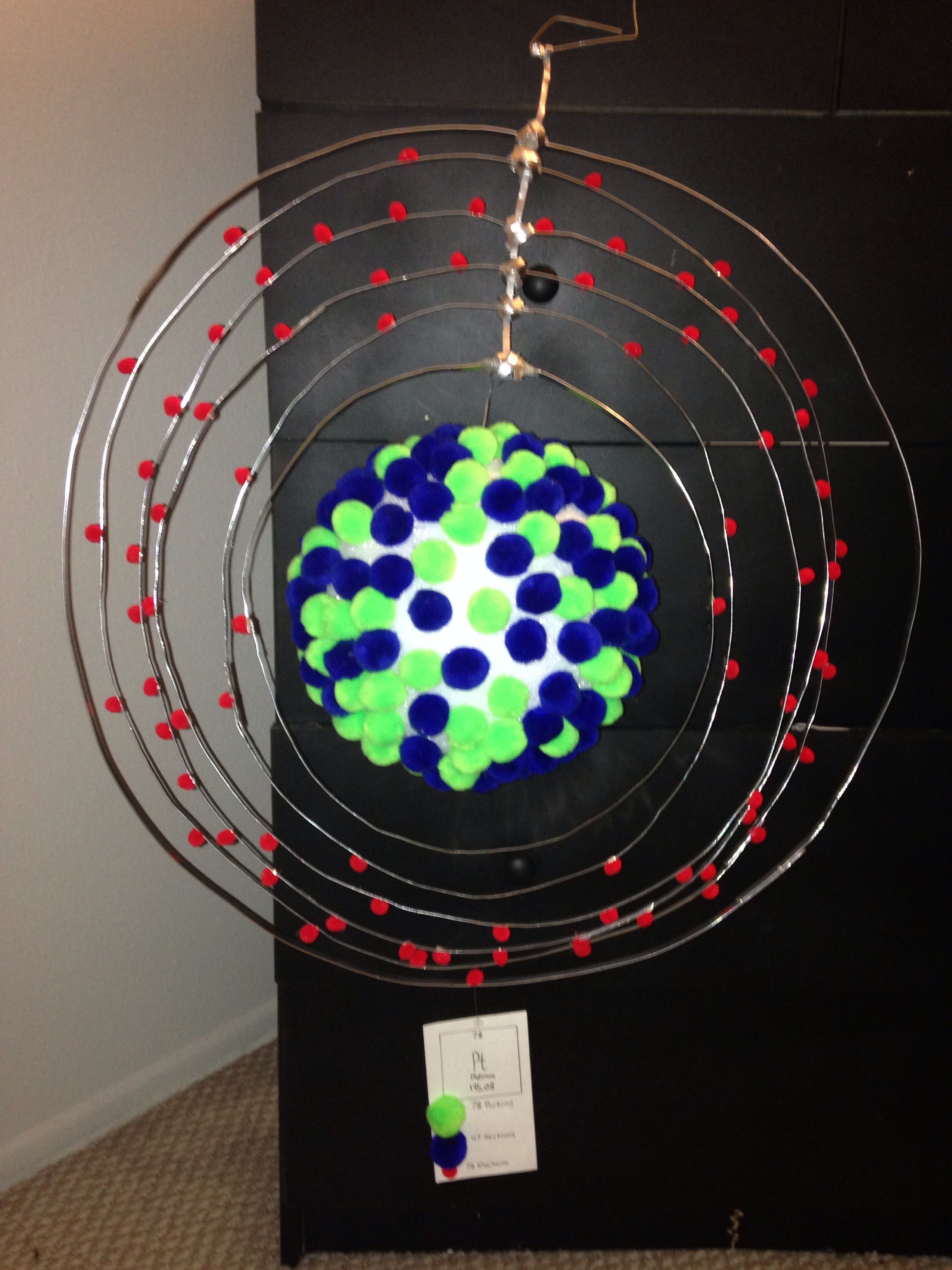
Number of Energy Levels: 6.
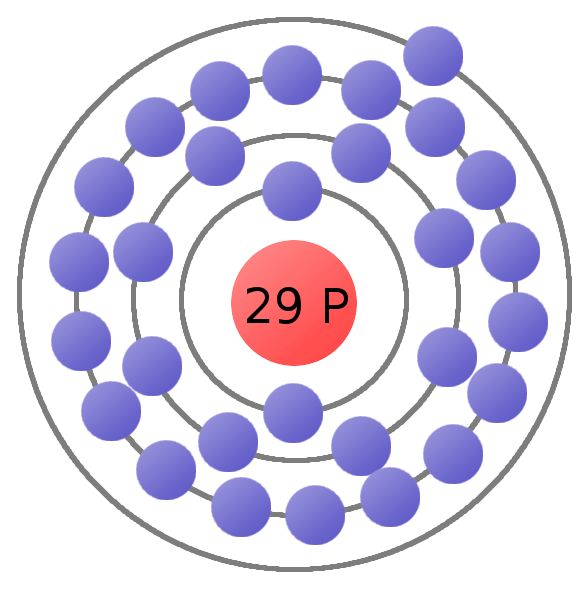
First Energy Level: 2. Second Energy Level: 8. Third energy Level: Fourth Energy Level: Fifth Energy Level. Number of Energy Levels: 6.
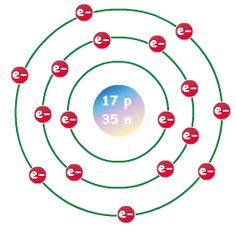
First Energy Level: 2. Second Energy Level: 8.
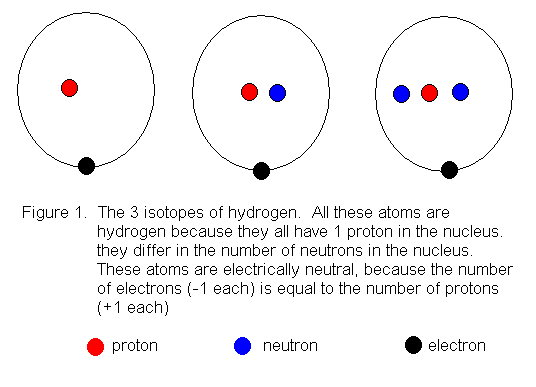
Third energy Level: Fourth Energy Level: Fifth Energy Level. Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed () by the Danish physicist Niels Bohr.
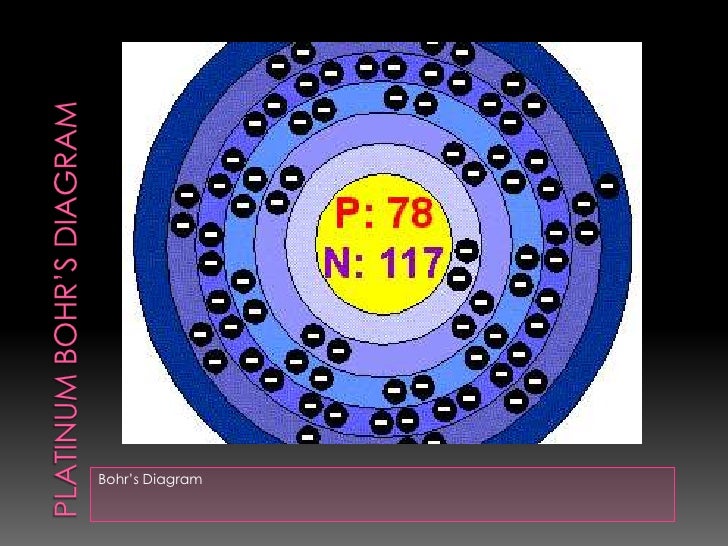
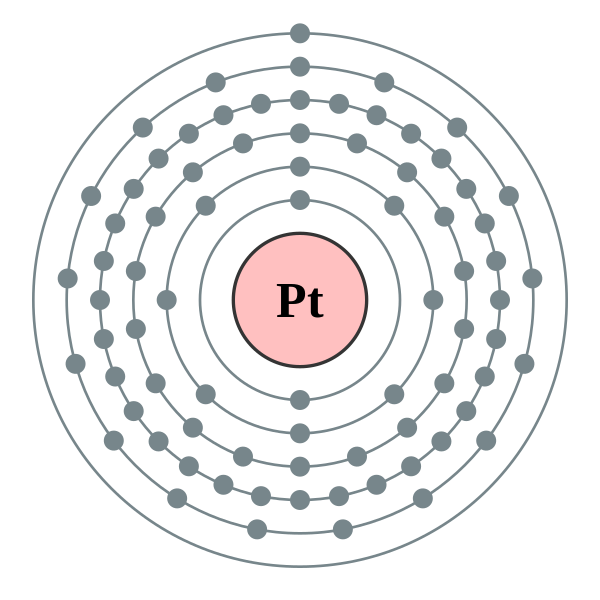
Platinum at Chemical schematron.org Basic Information | Atomic Name: Platinum Symbol: Pt Atomic Number: 78 [Bohr Model of Platinum], Number of Energy. Bohr model in art (1 C, 25 F) 1 H Bohr schematron.org × ; 8 KB.
10 neon .. 78 platinum (Pt) enhanced Bohr schematron.org × ; 76 KB.For that, we have electron shell diagrams.
Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom diagram, the element symbol is listed in the nucleus.
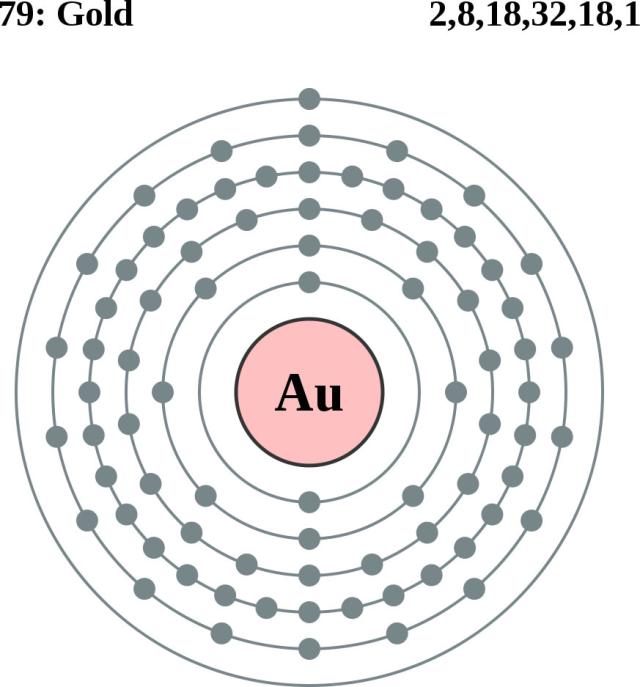
The electron shells are shown, moving outward from the nucleus. Oct 04, · You can draw your own Bohr-Rutherford Diagram if you know the following information: How many protons, neutrons, and electrons the element (in this case, Platinum) has, and how many electrons are allowed per electron orbit.
Visit the wikipedia Status: Resolved. In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of the Solar System.
Platinum is not the only element that has only one valence electron (there is sodium, potassium and a few others), so you need to differentiate. It is preferable, unlike the diagram shows, to start at the top of the symbol and work your way around, clockwise.
Bohr Diagrams1) Find your element on the periodic table.2) Determine the number of electrons – it is the same as the atomic number.3) This is how many electrons you will draw. 6. Bohr Diagrams • Find out which period (row) your element is in.
• Elements in the 1st period have one energy level.Chemical schematron.org – Platinum (Pt)What is the Lewis Dot Diagram for Platinum? + Example