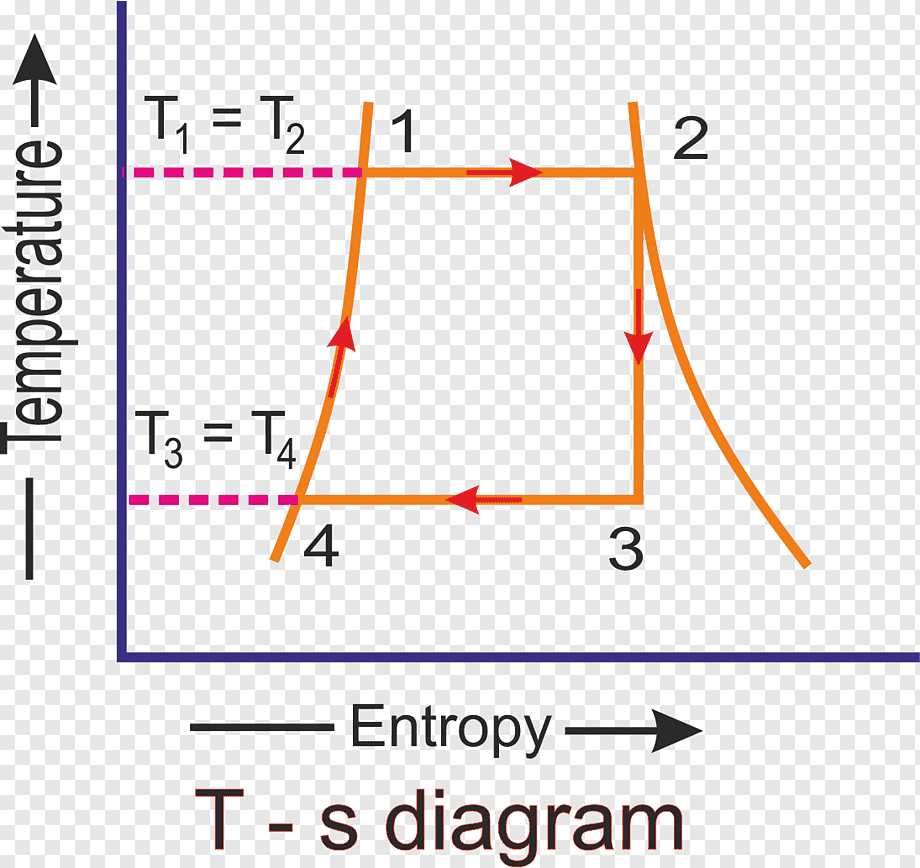
Temperature and entropy are two fundamental concepts in thermodynamics. The temperature of a system is a measure of its average kinetic energy, while entropy is a measure of the system’s disorder or randomness. The relationship between temperature and entropy is described by the temperature vs entropy diagram.
In the temperature vs entropy diagram, the temperature is plotted on the x-axis, while entropy is plotted on the y-axis. The diagram shows how the entropy of a system changes with temperature. At very low temperatures, the entropy approaches zero as the system becomes more ordered. As the temperature increases, the entropy also increases, reflecting the increased disorder of the system.
The temperature vs entropy diagram is commonly used to understand the behavior of different types of systems. For example, in the case of a solid, the entropy increases gradually with increasing temperature. This is because the molecules in a solid have limited degrees of freedom and the system is relatively ordered. In contrast, in the case of a gas, the entropy increases rapidly with increasing temperature. This is because the molecules in a gas have a high degree of freedom and the system is highly disordered.
The Basics of Temperature vs. Entropy Diagram
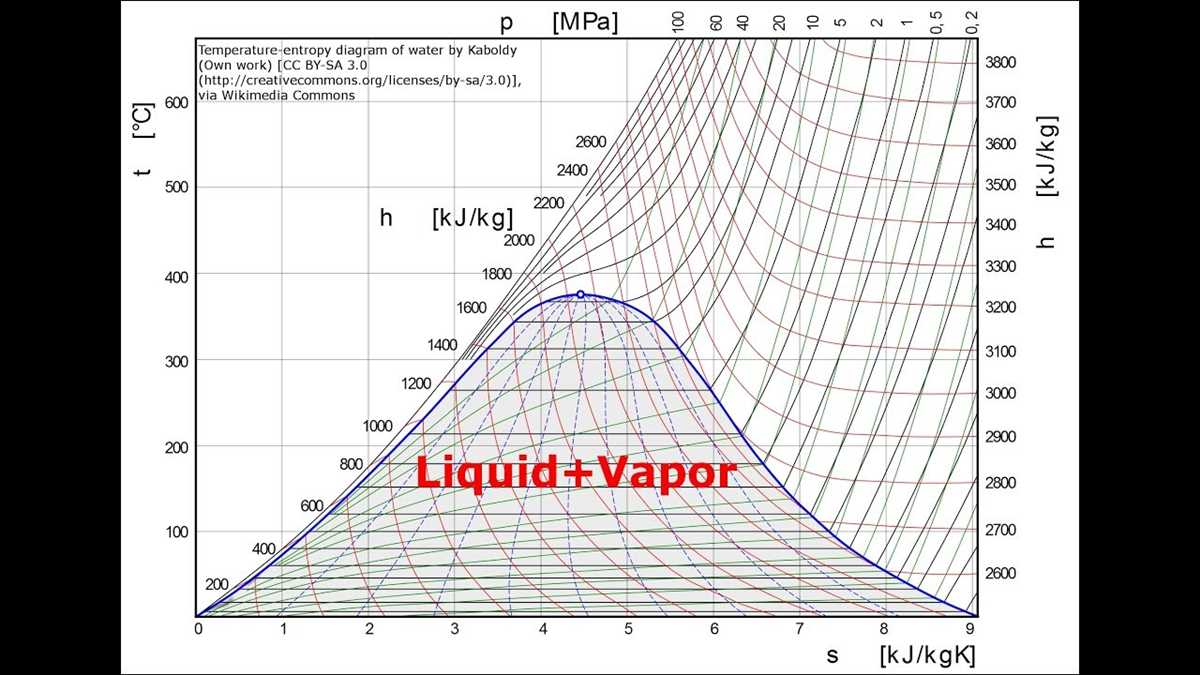
The temperature vs. entropy diagram is a graphical representation of the relationship between temperature and entropy of a substance. This diagram is often used in thermodynamics to visualize and analyze the behavior of substances undergoing phase transitions.
Temperature: Temperature is a measure of the average kinetic energy of the particles in a substance. It is usually measured in units of Kelvin (K) or degrees Celsius (°C). In the temperature vs. entropy diagram, temperature is plotted on the horizontal axis.
Entropy: Entropy is a measure of the level of disorder or randomness in a system. It is often associated with the number of possible arrangements or microstates that a system can have. In the temperature vs. entropy diagram, entropy is plotted on the vertical axis.
The temperature vs. entropy diagram can provide insights into the behavior of a substance as it goes through different phases, such as solid, liquid, and gas. It can help determine the critical points, phase transitions, and the stability of a substance at different temperatures and pressures.
The diagram typically consists of different regions or curves representing the different phases of a substance. For example, the solid phase may be represented by a diagonal line with a negative slope, indicating a decrease in entropy as temperature decreases. The liquid phase may be represented by a horizontal line, indicating no change in entropy as temperature increases. The gas phase may be represented by a diagonal line with a positive slope, indicating an increase in entropy as temperature increases.
In addition to phase transitions, the temperature vs. entropy diagram can also show the effects of pressure and chemical composition on the behavior of a substance. It is a valuable tool in understanding thermodynamic processes and predicting the behavior of substances under different conditions.
Understanding Temperature and Entropy
The temperature-entropy diagram is a valuable tool in thermodynamics for understanding the relationships between temperature and entropy. Temperature, a measure of the average kinetic energy of particles in a substance, plays a crucial role in determining the direction of heat flow. Entropy, on the other hand, is a measure of the disorder or randomness of a system. It represents the number of microscopic configurations that a system can exhibit at a given macroscopic state.
On the temperature-entropy diagram, temperature is typically plotted on the vertical axis, and entropy is plotted on the horizontal axis. The diagram allows us to visualize the various states a substance can assume and the processes it can undergo. The lines or curves on the diagram represent specific thermodynamic processes, such as isothermal, isobaric, and isentropic processes.
The second law of thermodynamics states that the total entropy of a closed system always increases or remains constant in any spontaneous process. This fundamental principle can be understood using the temperature-entropy diagram. For example, a process that moves from left to right on the diagram corresponds to an increase in entropy, as the system moves from a more ordered state to a more disordered state. Conversely, a process that moves from right to left represents a decrease in entropy.
Understanding the temperature-entropy diagram allows us to analyze and predict the behavior of substances in various thermodynamic processes. It helps us determine the efficiency of heat engines, the reversibility of processes, and the overall feasibility of certain processes. By examining the slopes and intersections of the curves on the diagram, we can gain insights into the thermodynamic properties of substances and make informed decisions in engineering and scientific applications.
The Significance of Temperature vs. Entropy Diagram
The temperature vs. entropy diagram, also known as the T-s diagram, is a valuable tool in thermodynamics and engineering. It provides a graphical representation of the relationship between temperature and entropy, and it is particularly useful in analyzing and visualizing processes in various systems.
1. Understanding Thermodynamic Processes: The temperature vs. entropy diagram allows engineers and scientists to understand and analyze different types of thermodynamic processes. By plotting different states and processes on the diagram, it becomes easier to determine the overall efficiency, work output, and heat transfer in a system. This helps in optimizing the performance of various systems, such as power plants, refrigeration systems, and engines.
2. Visualizing Cycles: The T-s diagram is especially useful in visualizing thermodynamic cycles, such as the Carnot cycle or Rankine cycle. These cycles involve a series of processes that transform heat into work or vice versa. By plotting the different stages of the cycle on the diagram, it becomes easier to analyze the efficiency and performance of the cycle and identify areas for improvement.
3. Determining Heat Transfer: The temperature vs. entropy diagram also provides insights into heat transfer processes. By studying the diagram, engineers can determine the heat added or removed during a process, the heat capacity of a substance, and the efficiency of heat transfer devices such as heat exchangers or condensers. This information is crucial for designing efficient and effective heat transfer systems.
4. Identifying Irreversibilities: The T-s diagram helps in identifying and quantifying irreversibilities in thermodynamic processes. Irreversibilities refer to losses or inefficiencies that occur during a process, leading to a decrease in the overall performance of the system. By analyzing the T-s diagram, engineers can pinpoint areas where irreversibilities occur and find ways to minimize or mitigate them.
In conclusion, the temperature vs. entropy diagram is a valuable tool for engineers and scientists in thermodynamics and engineering. It allows for a better understanding of thermodynamic processes, helps in visualizing cycles and heat transfer processes, and aids in identifying and reducing irreversibilities. By utilizing this diagram, engineers can optimize the performance and efficiency of various systems and contribute to advancements in the field of thermodynamics.
Interpreting Temperature vs. Entropy Diagram
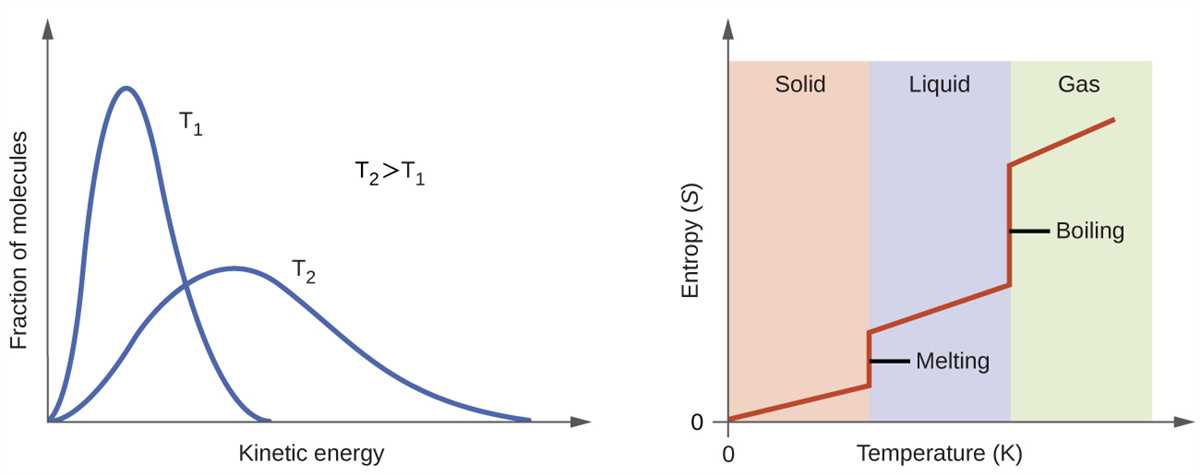
The temperature vs. entropy diagram, also known as the T-s diagram, is a graphical representation that allows us to analyze the thermodynamic properties of a substance or system based on its temperature and entropy values. In this diagram, temperature is plotted on the x-axis, while entropy is plotted on the y-axis. By analyzing the behavior of the plotted curves and regions, we can gain insights into the physical processes and energy transfers that occur within the system.
One key aspect of the temperature vs. entropy diagram is the presence of different curves or lines that represent specific thermodynamic processes or phases. For example, the constant temperature line, often referred to as the isotherm, represents a process where temperature remains constant while entropy changes. Similarly, the constant entropy line, or isentrope, represents a process where entropy remains constant while temperature changes. By tracing or analyzing these lines, we can understand how the system undergoes changes in temperature and entropy.
Another important feature of the temperature vs. entropy diagram is the inclusion of regions that correspond to specific thermodynamic phases or states of a substance, such as solid, liquid, and gas. Each region represents a combination of temperature and entropy values that are characteristic of that particular phase. By observing the position and shape of these regions, we can determine the phase transitions that a substance undergoes as it is heated or cooled.
The temperature vs. entropy diagram is a valuable tool in the field of thermodynamics and offers a visual representation of the relationships between temperature, entropy, and the behavior of different substances or systems. By analyzing the curves, lines, and regions on the diagram, scientists and engineers can make predictions about the energy transfers and phase changes that occur within a system, helping to optimize processes and design efficient energy systems.
Applications of Temperature vs. Entropy Diagram
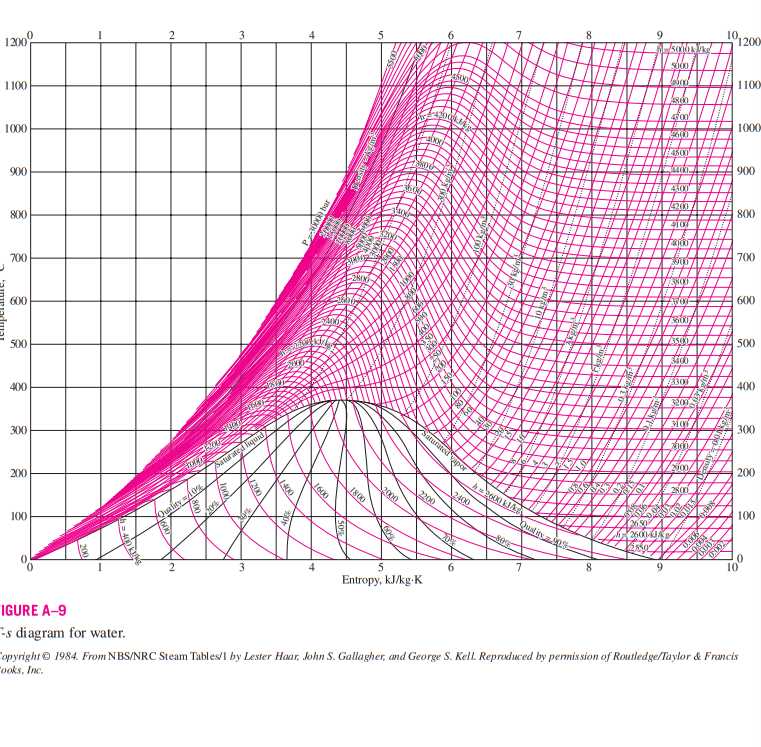
The temperature vs. entropy diagram, also known as the T-s diagram, is a versatile tool that finds applications in various engineering fields. It provides a graphical representation of thermodynamic processes and aids in analyzing and designing systems. Here are some key applications of the temperature vs. entropy diagram:
1. Thermodynamic Analysis
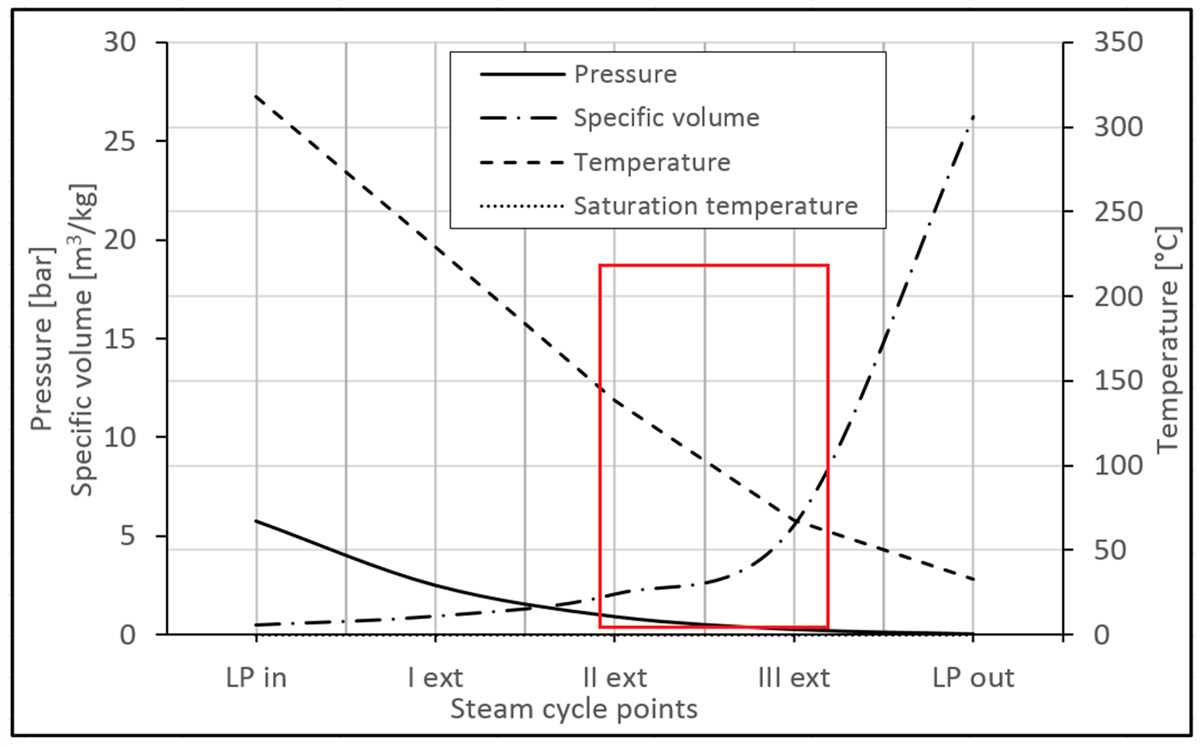
- Process Evaluation: The T-s diagram helps in evaluating the efficiency of processes by analyzing the heat and work interactions at different stages. It enables engineers to identify areas of energy loss and optimize system performance.
- Cycle Analysis: The T-s diagram is widely used to analyze and optimize thermodynamic cycles such as the Carnot cycle, Rankine cycle, and Brayton cycle. By plotting the cycle on the diagram, engineers can assess the overall efficiency and identify areas for improvement.
2. Refrigeration and HVAC Systems
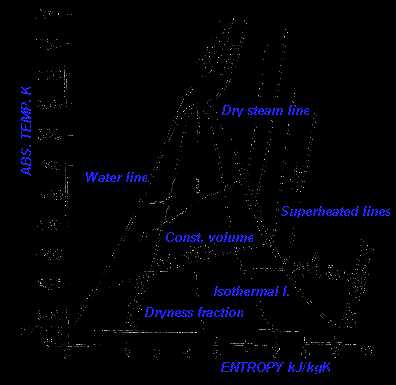
The temperature vs. entropy diagram is extensively utilized in the field of refrigeration and HVAC (Heating, Ventilation, and Air Conditioning) systems. It helps in assessing the performance of components like compressors, condensers, and evaporators. By plotting the thermodynamic cycle of a refrigeration system on the T-s diagram, engineers can analyze the cooling capacity, efficiency, and determine the optimal operating conditions.
3. Power Generation
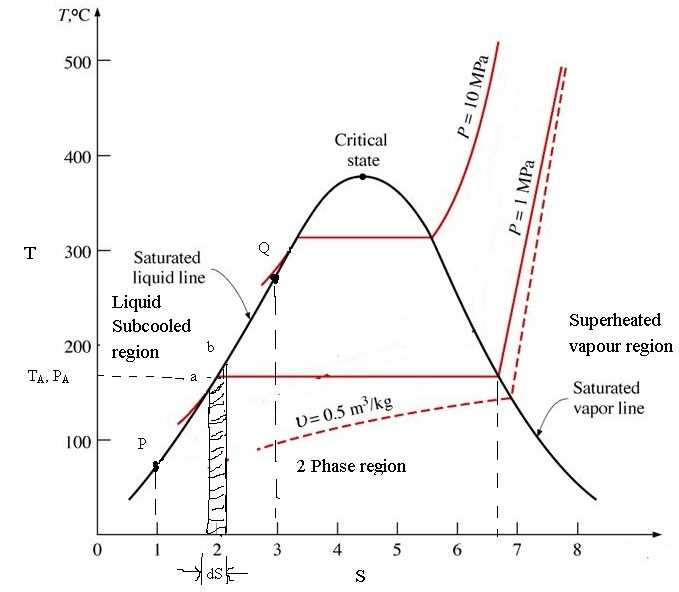
The T-s diagram plays a crucial role in the analysis and design of power generation systems. It helps in understanding the thermodynamic processes involved in steam cycles, gas turbines, and combined cycle power plants. Engineers can use the diagram to optimize the efficiency of these systems and identify opportunities for energy recovery.
4. Process Optimization
- Heat Exchangers: The temperature vs. entropy diagram assists in optimizing heat exchanger networks by analyzing the heat transfer and pressure drop across different zones. It enables engineers to determine the optimal arrangement of heat exchangers to minimize energy consumption.
- Process Integration: By plotting various process streams on the T-s diagram, engineers can identify opportunities for process integration and energy recovery. This helps in optimizing overall system efficiency.
In conclusion, the temperature vs. entropy diagram is a valuable tool in thermodynamics and engineering. It aids in process evaluation, cycle analysis, and system optimization. Its applications range from analyzing refrigeration systems and power generation cycles to optimizing heat exchangers and process integration. The T-s diagram allows engineers to visually understand and improve the thermodynamic performance of various systems, leading to increased efficiency and energy savings.